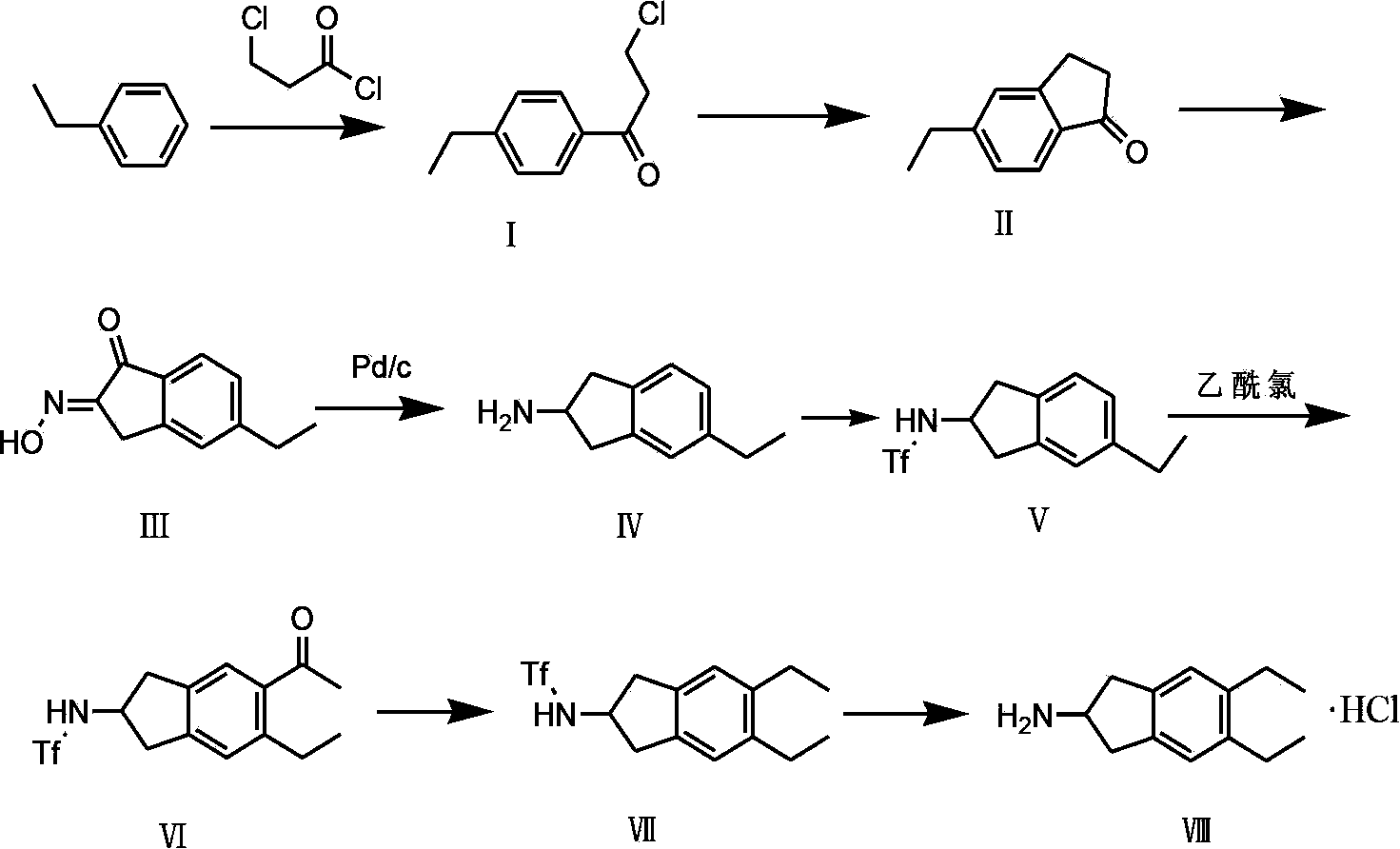
Preparation method of 5,6-diethyl-2,3-dihydro-1H-indene-2-amine hydrochloride
A technology of amine hydrochloride and diethyl, applied in the field of synthesis of pharmaceutical intermediates, can solve the problems of industrial production limitation, high price and the like, and achieve the effects of easy industrialization and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] A preparation method of 5,6-diethyl-2,3-dihydro-1H-indene-2-amine hydrochloride, the steps are as follows:
[0023] (1) Synthesis of compound Ⅰ
[0024] Put 780g of aluminum trichloride and 8L of dichloromethane into a 10L three-necked flask, cool to -1-1°C, add 600g of 3-chloropropionyl chloride dropwise, react for 30min after dropping, and then add 500g ethylbenzene dropwise, after the addition is complete Warm up to room temperature (20-25℃) and react for 8 hours, then pour the reactants into 10L 1M hydrochloric acid ice water (hydrochloric acid ice water mixture) to extract, extract with dichloromethane, wash with saturated sodium bicarbonate once, and wash once with water. The product was dried with sodium sulfate, spin-dried, then freeze-crystallized, and dried to obtain 847 g of solid with a melting point of 63-64°C. The product was confirmed by nuclear magnetism and the yield was 90%.
[0025] (2) Synthesis of compound Ⅱ
[0026] Put 1.5L of 98% concentrated sulfuric a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com