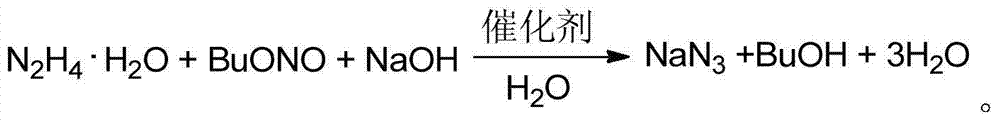Aqueous-phase synthetic method of sodium azide with n-butyl alcohol circularly recycled
A sodium azide water, recycling technology, applied in the direction of azide acid/azide/halogen azide, organic chemistry, nitrite preparation, etc., can solve the problems of gas leakage and achieve low cost and high yield high efficiency and reduce environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 (sodium azide is synthesized):
[0024] In a 1000mL round bottom flask, add sodium hydroxide (40g, 1mol) and dissolve it in 80ml of water (4.44mol), add hydrazine hydrate (1mol), add methanol (3.2g, 0.1mol), add dropwise butyl nitrite Ester (113.4 g, 1.1 mol). Take it out after reacting at room temperature 25°C for 12 hours, recover n-butanol by distillation under reduced pressure, and filter to obtain a white solid. In the white solid, the free alkali content obtained by inspection is 0.85%, and sodium azide is 97.07%, that is, the white solid contains sodium azide The mass percent is 97.07%, that is, the purity is 97.07%, and the yield is 91%.
Embodiment 2
[0025] Embodiment 2 (sodium azide is synthesized):
[0026] Add sodium hydroxide (40g, 1mol) and dissolve in 80 ml of water (4.44mol) in a 1000mL round bottom flask, add hydrazine hydrate (1mol), add tetrabutylammonium chloride (0.06g, 0.0002mol), Butyl nitrite (113.4 g, 1.1 mol) was added dropwise. React at room temperature 25°C for 12 hours and take it out. Distill under reduced pressure to recover n-butanol, filter to obtain a white solid, check the free base content of 1.11%, sodium azide 97.35%, and the yield is 94%.
Embodiment 3
[0027] Embodiment 3 (sodium azide is synthesized):
[0028] Add sodium hydroxide (40g, 1mol) in 1000mL round bottom flask and dissolve in 80ml of water (4.44mol), add hydrazine hydrate (1mol), add tetrabutylammonium bromide (40g, 0.0002mol), drop Add butyl nitrite (113.4 g, 1.1 mol). React at room temperature 25°C for 12 hours and take it out. Recover n-butanol by distillation under reduced pressure, and filter to obtain a white solid. The free base content of the obtained product is 4.08%, sodium azide is 94.48%, and the yield is 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com