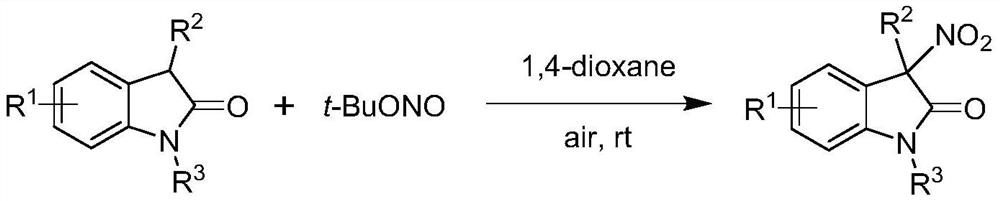
Method for preparing chiral 3-nitroindole compound through nickel-catalyzed asymmetric nitration reaction
A technology of nitroindole and nitration reaction, which is applied in the field of preparation of chiral 3-nitroindole compounds, can solve the problems of uncontrollable reaction system, unstable nitration reagent, and low reaction efficiency, and reach the scope of application of substrates Wide range of effects, high reaction stereoselectivity, and low catalyst cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] A method for preparing chiral 3-nitroindole compounds (Ⅲa) through nickel-catalyzed asymmetric nitration reaction, the reaction scheme is as follows:
[0040]
[0041] Concrete preparation steps are as follows: add indol-2-ketone compound (being raw material Ia, 62mg, 0.2mmol) in 5mL round bottom flask, tert-butyl nitrite (being raw material II, 31mg, 0.3mmol), nickel catalyst ( NiCl 2 DME, 3.3mg, 0.015mmol), Box ligand (7mg, 0.016mmol), tert-butanol (15mg, 0.2mmol), 100 mg of molecular sieve and 1 mL of dichloromethane were then stirred and reacted in an oxygen atmosphere at 0° C., and the reaction progress was detected by TLC until the raw material Ia disappeared (reaction time was 12 h). After the reaction was completed, the solvent was removed from the resulting reaction solution, and the resulting crude product was separated and purified by column chromatography (eluent: ethyl acetate:petroleum ether=1:50~1:20, v / v) to obtain 34 mg of the target product. The y...
Embodiment 2
[0047] A method for preparing chiral 3-nitroindole compound (Ⅲb) through nickel-catalyzed asymmetric nitration reaction, the reaction scheme is as follows:
[0048]
[0049] The specific preparation steps are as follows: in the 5mL round bottom flask, add indol-2-ketone compound (i.e. raw material Ib, 65mg, 0.2mmol), tert-butyl nitrite (i.e. raw material II, 31mg, 0.3mmol), nickel catalyst ( NiCl 2 DME, 3.3mg, 0.015mmol), Box ligand (7mg, 0.016mmol), tert-butanol (15mg, 0.2mmol), 100 mg of molecular sieve and 1 mL of dichloromethane were then stirred and reacted in an oxygen atmosphere at 0° C., and the reaction progress was detected by TLC until the raw materials disappeared (reaction time was 12 h). After the reaction was completed, the solvent was removed from the resulting reaction solution, and the resulting crude product was separated and purified by column chromatography (eluent: ethyl acetate:petroleum ether=1:50~1:20, v / v) to obtain 39 mg of the target product. T...
Embodiment 3
[0055] A method for preparing chiral 3-nitroindole compound (Ⅲc) through nickel-catalyzed asymmetric nitration reaction, the reaction scheme is as follows:
[0056]
[0057] Concrete preparation steps are as follows: in the 5mL round-bottomed flask, add indol-2-ketone compound (being raw material Ic, 84mg, 0.2mmol), tert-butyl nitrite (being raw material II, 31mg, 0.3mmol), nickel catalyst ( NiCl 2 DME, 3.3mg, 0.015mmol), Box ligand (7mg, 0.016mmol), tert-butanol (15mg, 0.2mmol), 100 mg of molecular sieve and 1 mL of dichloromethane were then stirred and reacted in an oxygen atmosphere at 0° C., and the reaction progress was detected by TLC until the raw materials disappeared (reaction time was 12 h). After the reaction was completed, the resulting reaction solution was removed from the solvent, and the resulting crude product was separated and purified by column chromatography (eluent: ethyl acetate:petroleum ether=1:50~1:20, v / v) to obtain 65 mg of the target product, w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com