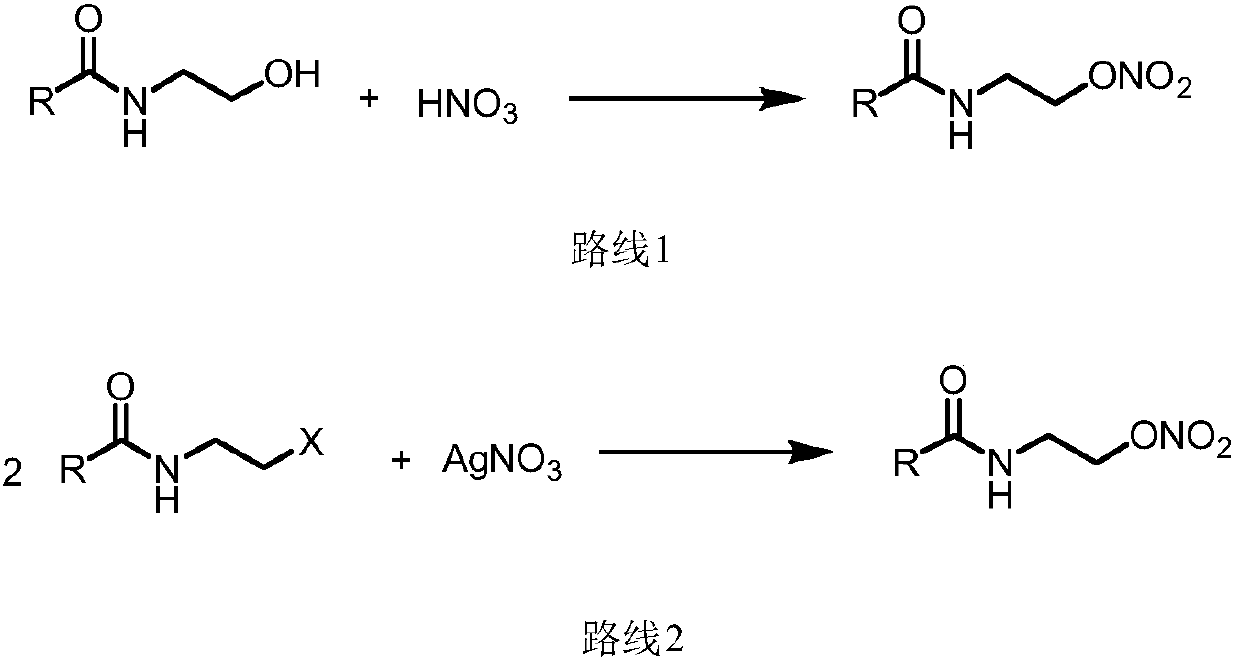
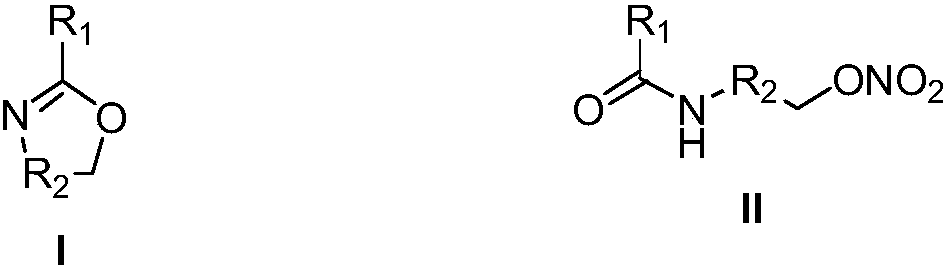Method for preparing nitrate ester compound by adopting micro-flow field reaction technology
A technology for compounds and nitrates, which is applied in the field of preparing nitrate compounds by using microfluidic field reaction technology, can solve the problems of intractable treatment of acid wastewater, inability to enlarge industrially, expensive equipment investment, etc., and achieves easy post-processing, improved safety, The effect of shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] 2-Phenyl-4,5-dihydrooxazole (2mmol, 1.0eq) and TBN (6mmol, 3.0eq) were respectively dissolved in 20mL 1,4-dioxane. Take the prepared solvent and load it in a syringe, and pump it into the microchannel modular reaction device. The reaction solution was reacted at 80°C, and the reaction time was 2h. The reaction solution flowing out of the microchannel modular reaction device was poured into saturated sodium sulfite solution (30mL), extracted with ethyl acetate (50mL×3), and the combined organic layer was washed with anhydrous Na 2 SO 4 Dry, filter, and evaporate the solvent in a vacuum to obtain a crude product. Recrystallize the crude product with ethanol to obtain a pure product with a yield of 95%.
Embodiment 2
[0056] 2-(p-methylphenyl)-4,5-dihydrooxazole (2mmol, 1.0eq) and TBN (6mmol, 3.0eq) were dissolved in 20mL of 1,4-dioxane. Take the prepared solvent and load it in a syringe, and pump it into the microchannel modular reaction device. The reaction solution was reacted at 80°C, and the reaction time was 2h. The reaction solution flowing out of the microchannel modular reaction device was poured into saturated sodium sulfite solution (30mL), extracted with ethyl acetate (50mL×3), and the combined organic layer was washed with anhydrous Na 2 SO 4 Dry, filter, and evaporate the solvent in a vacuum to obtain a crude product. The crude product is recrystallized from ethanol to obtain a pure product with a yield of 91%.
Embodiment 3
[0060] Dissolve 2-(4-chlorophenyl)-4,5-dihydrooxazole (2mmol, 1.0eq) and TBN (6mmol, 3.0eq) in 20mL 1,4-dioxane respectively. Take the prepared solvent and load it in a syringe, and pump it into the microchannel modular reaction device. The reaction solution was reacted at 80°C, and the reaction time was 2h. The reaction solution flowing out of the microchannel modular reaction device was poured into saturated sodium sulfite solution (30mL), extracted with ethyl acetate (50mL×3), and the combined organic layer was washed with anhydrous Na 2 SO 4 Dry, filter, and evaporate the solvent in a vacuum to obtain a crude product. Recrystallize the crude product with ethanol / flash column chromatography to obtain a pure product with a yield of 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com