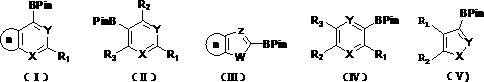
Method for continuously synthesizing arylboronic acid ester by utilizing microreactor
An aryl borate, microreactor technology, applied in chemical instruments and methods, compounds containing periodic table Group 3/13 elements, organic chemistry, etc., to achieve the effect of maintaining reaction temperature, easy control, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
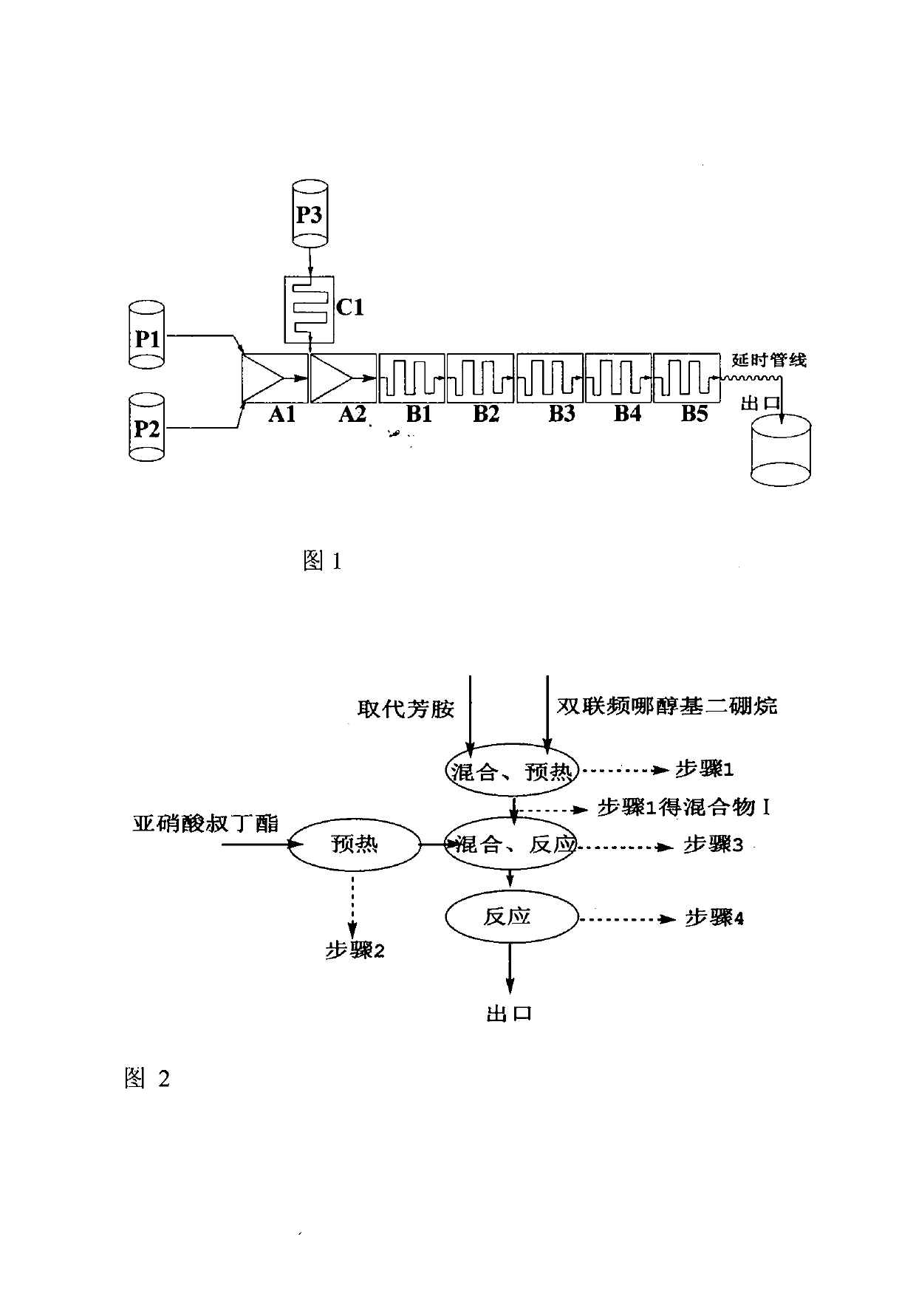
[0042] (1) The device used: a continuous microchannel reactor, refer to figure 1 The system device diagram determines the connection mode of the microreactor modules, the number of mixed reaction modules and the length of the delay pipeline are determined according to the flow rate and reaction residence time, and the heat exchange medium is heat transfer oil;
[0043] (2) Mixture I configuration: pre-configure the acetonitrile solution of 2-fluoro-4-thiamphenicol aniline (0.5mol / L), the acetonitrile solution of double pinacolyl diborane (1.0mol / L), set Set the flow rate ratio of metering pump P1 and pump P2 to 2-fluoro-4-thiamphenicol aniline: double pinacol diborane = 1.0: 0.55, and pump them into the mixing module A1 at the same time, set the heat exchange in this section When the temperature of the mixer is 80°C, the uniformly mixed mixture I can be prepared. At this moment, the mol ratio of 2-fluoro-4-thiamphenicol aniline: double pinacol base diborane is 1:1.1;
[004...
Embodiment 2
[0047] (1) The device used: a continuous microchannel reactor, refer to figure 1 The system device diagram determines the connection mode of the microreactor modules, the number of mixed reaction modules and the length of the delay pipeline are determined according to the flow rate and reaction residence time, and the heat exchange medium is heat transfer oil;
[0048] (2) Mixture I configuration: pre-configure the acetonitrile solution of 3-fluoro-4-thiamphenicol aniline (0.5mol / L), the acetonitrile solution of double pinacolyl diborane (1.0mol / L), set Set the flow rate ratio of the metering pump P1 and pump P2 to 3-fluoro-4-thiamphenicol aniline: double pinacol diborane = 1.0 : 0.55, and pump them into the mixing module A1 at the same time, set the heat exchange in this section When the temperature of the mixer is 80°C, the uniformly mixed mixture I can be prepared. At this moment, the mol ratio of 3-fluoro-4-thiamphenicol aniline: double pinacol base diborane is 1:1.1;
...
Embodiment 3
[0052] (1) The device used: a continuous microchannel reactor, refer to figure 1 The system device diagram determines the connection mode of the microreactor modules, the number of mixed reaction modules and the length of the delay pipeline are determined according to the flow rate and reaction residence time, and the heat exchange medium is heat transfer oil;
[0053](2) Mixture I configuration: pre-configure the acetonitrile solution of 2-chloroaniline (1.0mol / L), the acetonitrile solution of double pinacol diborane (1.0mol / L), set the metering pump P1, pump The flow rate ratio of P2 is 2 chloroaniline: double-linked pinacol diborane = 0.5:0.55, and it is pumped into the mixing module A1 at the same time, and the temperature of the heat exchanger in this section is set to 80°C to prepare a well-mixed mixture I. Now 2-chloroaniline: the mol ratio of double pinacol base diborane is 1:1.1;
[0054] (3) The acetonitrile solution (1.0mol / L) of tert-butyl nitrite is pre-configu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com