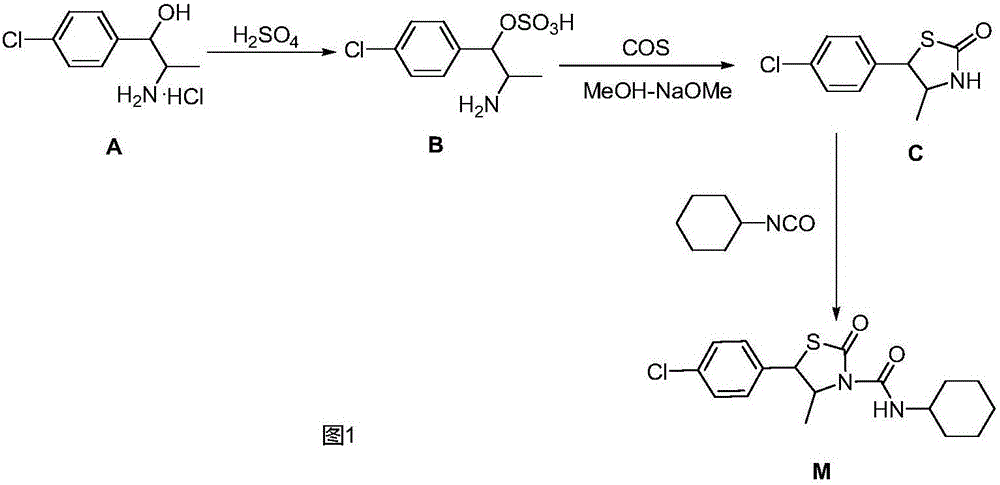
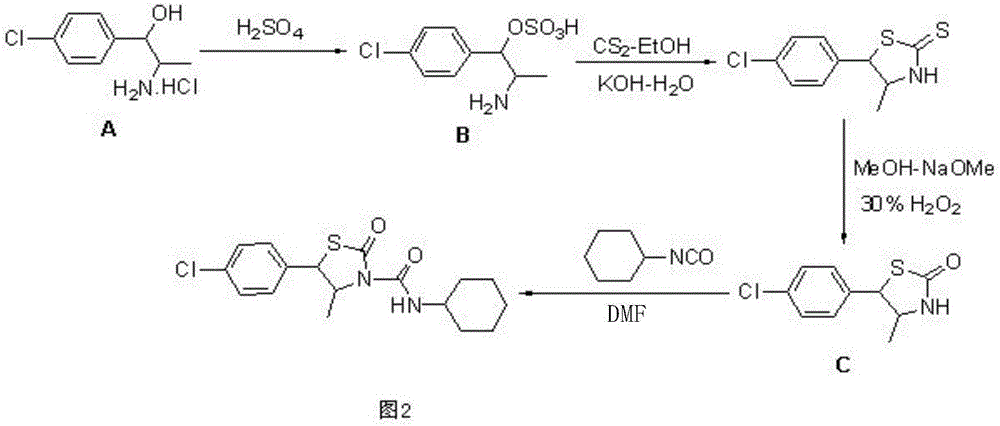
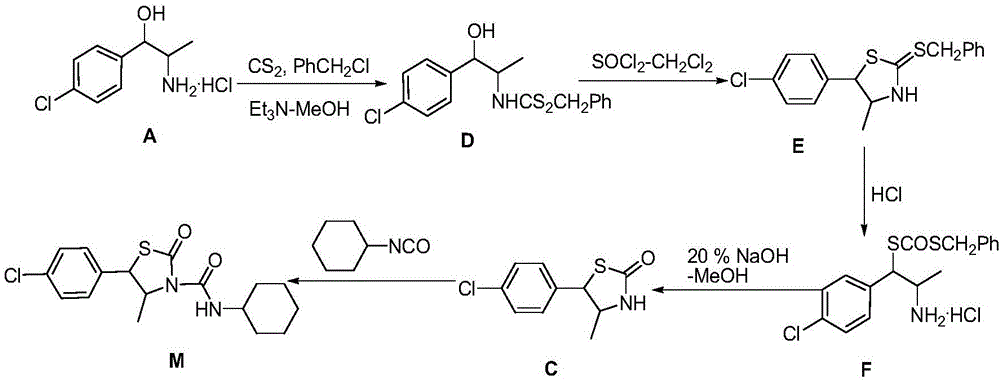
Hexythiazox and preparation method thereof
A technology of hexythifen and ketoxime is applied in the field of hexythifen and its preparation, and achieves the effects of improving reaction yield, good permeability and remarkable effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] A preparation method of hexythiazox, comprising the following steps:
[0043] Step S1, ketoximation reaction
[0044] The reaction equation is:
[0045]
[0046] Raw materials: p-chloropropiophenone, purity 99%; n-butyl nitrite, purity 99%; petroleum ether, temperature 90°C-120°C;
[0047] Operation: Put 350Kg p-chloropropiophenone and 700Kg petroleum ether into a 3000-liter reactor, cool down to 10°C, then add 235Kg n-butyl nitrite, stir for 5 hours, then cool down to 0°C, keep stirring for 0.5h, and discharge , filtered, washed with petroleum ether, and dried to obtain a solid ketoxime;
[0048] Step S2, alcohol amination reaction
[0049] The reaction equation is:
[0050]
[0051] Raw materials: ketoxime, purity 99%; methanol, purity 99.5%; hydrochloric acid, purity 30%; ethanol, purity 99.5%;
[0052] Operation: Put 180Kg of the prepared ketoxime and 480Kg of methanol into a 2000-liter reactor, add 1Kg of catalyst platinum, dissolve for 0.5h, replace wit...
Embodiment 2
[0069] A preparation method of hexythiazox, comprising the following steps:
[0070] Step S1, put 174Kg p-chloropropiophenone and 350Kg petroleum ether into a 3000-liter reactor, cool down to 20°C, then add 117.5Kg n-butyl nitrite, stir for 10h, then cool down to 0°C, keep stirring for 1.5h, Blowing, filtering, washing with petroleum ether, and drying to obtain solid ketoxime;
[0071] Step S2, put 90Kg of the prepared ketoxime and 240Kg of methanol into a 2000-liter reactor, add 1Kg of catalyst palladium, dissolve for 0.5h, replace 4 times with nitrogen, then replace 4 times with hydrogen, heat up to 40°C, and pass in hydrogen, Control the hydrogen pressure to 0.08Mpa until no more hydrogen can be added. After the hydrogen flow is completed, the catalyst palladium is removed by filtration while it is hot to obtain a methanol solution of the alcohol amine. The pH value is adjusted to 6.5 with hydrochloric acid, and the solvent is removed under reduced pressure to obtain the al...
Embodiment 3
[0076] A preparation method of hexythiazox, comprising the following steps:
[0077] Step S1, put 420Kg of p-chloropropiophenone and 840Kg of petroleum ether into a 3000-liter reactor, cool down to 15°C, then add 282Kg of n-butyl nitrite, stir for 7.5h, then cool down to 0°C, keep stirring for 1h, and let it cool down to 15°C. material, filtered, washed with petroleum ether, and dried to obtain a solid ketoxime;
[0078] Step S2, put 216Kg of the prepared ketoxime and 576Kg of methanol into a 2000-liter reactor, add 1.8Kg of catalyst nickel, dissolve for 0.5h, replace with nitrogen 3 times, then replace with hydrogen 3 times, heat up to 37°C, and pass in hydrogen , control the hydrogen pressure to 0.05Mpa, until no more hydrogen is added, the hydrogen flow is completed, the catalyst nickel is removed by filtration while it is hot, and the methanol solution of the alcohol amine is obtained, the pH value is adjusted to 6.3 with hydrochloric acid, and the solvent is removed under...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com