Nitric oxide donor based on sodium alginate as well as synthesis method and application of nitric oxide donor
A technology of sodium alginate and nitric oxide, which is applied to medical preparations containing active ingredients, prostheses, drug combinations, etc., and can solve the problems of prolonging the release time of nitric oxide, poor solubility of small molecule donors, and Over-fast release and other problems, to achieve the effect of increasing bioavailability, simple synthesis method, and long release time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Dissolve 0.2 g of sodium alginate in 20 mL of ultrapure water, add a final concentration of 50 mMEDC·HCl, and react for 1 h at room temperature to obtain solution A.
[0041] (2) Advocating N 2 5min, weigh L-cysteine hydrochloride with twice the molar amount of sodium alginate and add it to solution A in step (1), adjust the pH of the solution to 5 with 1M NaOH, and react at 25°C for 12h in the dark to obtain Reaction solution B.
[0042] (3) The reaction solution B of step (2) was placed in a dialysis bag with MWCO=3500Da, and the ultrapure water was changed every 12h, and the solution was dialyzed at room temperature for 48h, and the solution C was obtained after the dialysis.
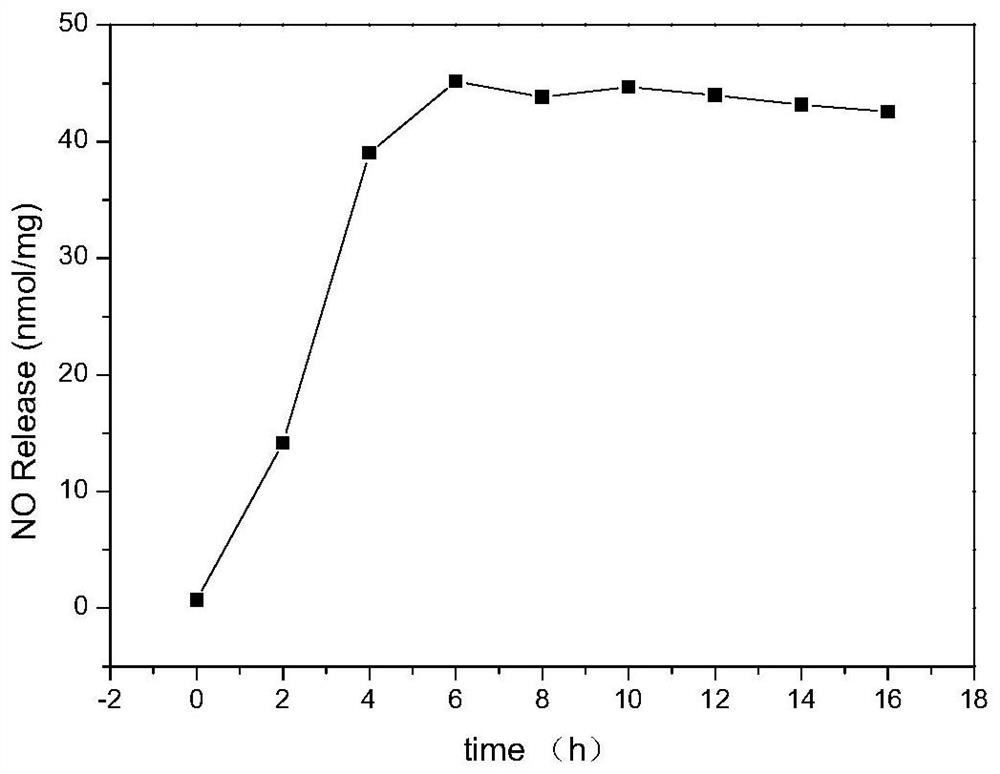
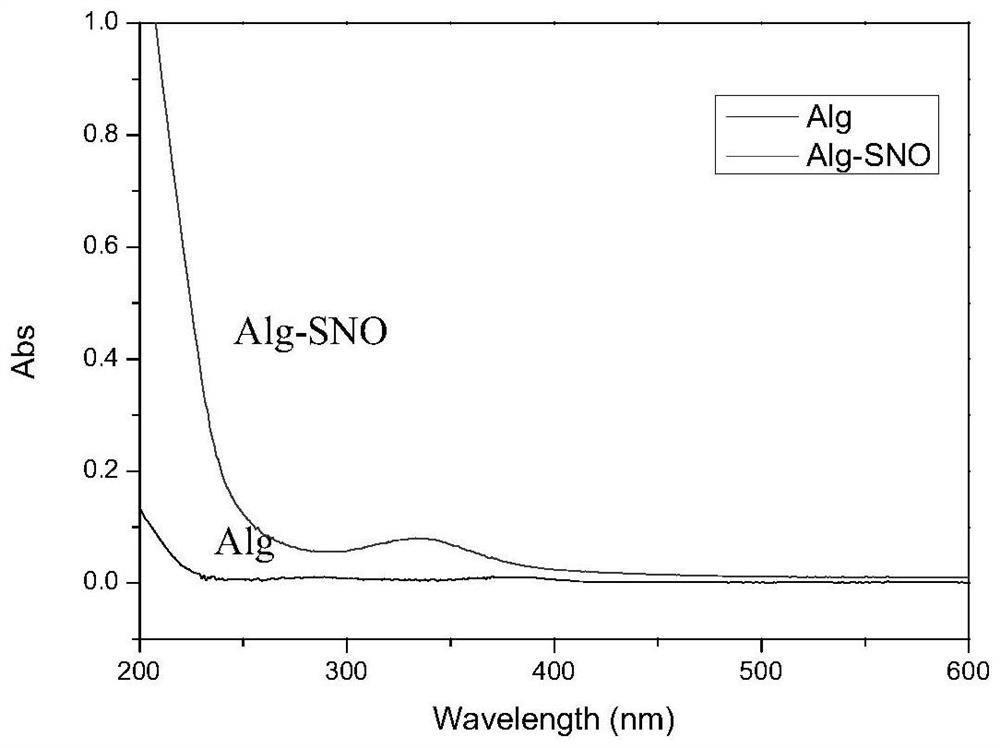
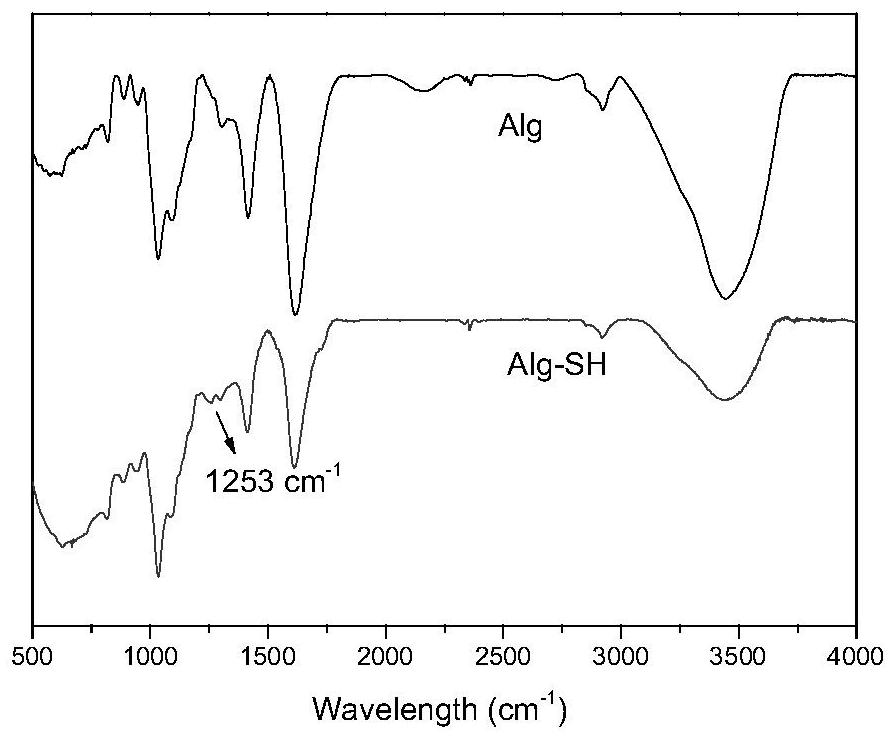
[0043] The sulfhydryl grafting rate on sodium alginate in solution C was measured by Ellman method, and solution C was freeze-dried to obtain sulfhydrylated sodium alginate. Through infrared solid tableting operation experiments, such as image 3 As shown, infrared spectroscopy charact...
Embodiment 2
[0048] (1) Dissolve 0.2 g of sodium alginate in 20 mL of ultrapure water, add a final concentration of 50 mMEDC·HCl, and react for 1 h at room temperature to obtain solution A.
[0049] (2) Advocating N 2 5min, weigh L-cysteine hydrochloride with twice the molar amount of sodium alginate and add it to solution A in step (1), adjust the pH of the solution to 6 with 1M NaOH, and react at 25°C for 12h in the dark to obtain Reaction solution B.
[0050] (3) The reaction solution B of step (2) is placed in the dialysis bag of MWCO=3500Da, and the ultrapure water is changed every 12h, and the dialysis is performed for 48h. Thiol grafting ratio.
[0051] (4) Directly adding tert-butyl nitrite with five times the molar amount of mercapto into solution C, adjusting the pH of the solution to 9, reacting at room temperature under nitrogen protection for 12h, using a dialysis bag with a molecular weight cut-off of 3500Da to dialyze with ultrapure water for 48h, Freeze drying to obta...
Embodiment 3
[0053] (1) Dissolve 0.2 g of sodium alginate in 20 mL of ultrapure water, add a final concentration of 50 mMEDC·HCl, and react for 1 h at room temperature to obtain solution A. (2) Advocating N 2 5min, weigh twice the molar amount of sodium alginate cystamine dihydrochloride and add it to solution A in step (1), adjust the pH of the solution to 6 with 1M NaOH, and react at 25°C for 12h in the dark to obtain reaction solution B .
[0054] (3) The reaction solution B of step (2) was placed in a dialysis bag with MWCO=3500Da, the water was changed every 12h, and the solution was dialyzed for 48h, and the solution C was obtained after the dialysis.
[0055] (4) DTT with a final concentration of 10 mM was added to solution C, the pH of the solution was adjusted to 7.5, and after 12 h of reaction, ultrapure water was used for dialysis for 48 h, the water was changed every 12 h, and solution D was obtained after dialysis.
[0056] (5) adopt the Ellman method to measure the sulfhyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com