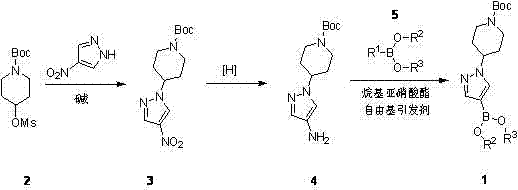
Method for synthesizing Crizotinib intermediate
A technology for crizotinib and intermediates, applied in the field of synthesizing crizotinib intermediates, can solve the problems of high cost, high preparation cost of reaction substrate iodide, difficult industrialized production and the like, and achieves reduction in preparation cost, It is convenient for industrial production and application, and the preparation cost is low.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045]
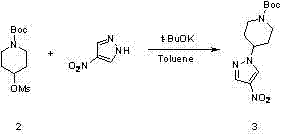
[0046] Add 4-nitropyrazole (3.73 g, 0.033 mol) and 80 mL of N,N-dimethylformamide into the reactor. Cool in an ice bath to 0°C, stir, add sodium hydride (0.93 g, 0.038 mol) in batches, stir at the same temperature for 1 h after addition, add compound 2 (10.0 g, 0.036 mol) to the reaction solution, heat up to 100°C, and react for 12 h . Cool, add 400 mL of water, extract three times with 400 mL of ethyl acetate, dry over anhydrous sodium sulfate, filter and spin dry to obtain the crude product, recrystallize from ethyl acetate petroleum ether to obtain the compound 3 (7.8 g yield 80%).
Embodiment 2
[0048]
[0049] Add 4-nitropyrazole (3.73 g, 0.033 mol) and 100 mL of dimethyl sulfoxide to the reactor. Cool in an ice bath to 0°C, stir, add potassium hydroxide (0.93 g, 0.038 mol) in batches, stir at the same temperature for 1 h after addition, add compound 2 (10 g, 0.036 mol) to the reaction solution, raise the temperature to 80°C, and react 18 h. Cool, add 400 mL of water, extract three times with 400 mL of ethyl acetate, dry over anhydrous sodium sulfate, filter and spin dry to obtain the crude product, recrystallize from ethyl acetate petroleum ether to obtain the compound 3 (7.1 g yield 72%).
Embodiment 3
[0051]
[0052] Add 4-nitropyrazole (3.73 g, 0.033 mol) and 80 mL of toluene into the reactor. Cool in an ice bath to 5 °C, stir, add potassium tert-butoxide (0.93 g, 0.038 mol) in batches, stir at the same temperature for 1 h after addition, add compound to the reaction solution 2 (10 g, 0.036 mol), heated up to 108°C, and reacted for 10 h. Cool, add 400 mL of water, separate the layers, extract three times with 400 mL of ethyl acetate, dry over anhydrous sodium sulfate, filter and spin dry to obtain the crude product, recrystallize from ethyl acetate / petroleum ether to obtain the compound 3 (7.4 g yield 76%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com