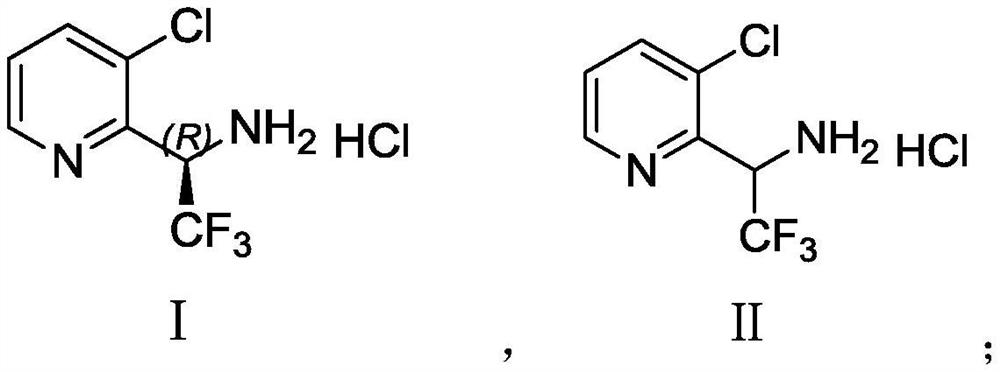
Synthesis method of (R)-3-chloropyridyl-2-trifluoroethylamine hydrochloride
A technology of trifluoroethylamine hydrochloride and chloropyridyl, which is applied in the field of synthesis of -3-chloropyridyl-2-trifluoroethylamine hydrochloride, can solve problems such as limited quantity, achieve less waste and improve Effect of conversion rate and safety, availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
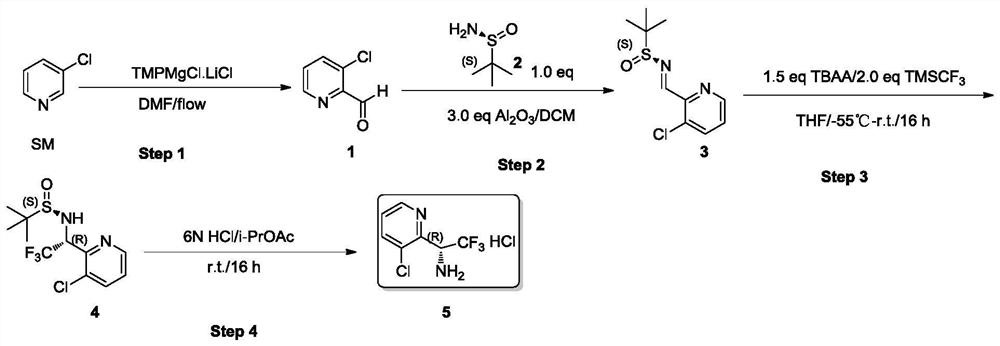
[0069] In this example, the racemate 3-chloropyridyl-2-trifluoroethylamine hydrochloride was prepared through the following steps, and the reaction scheme is as follows:
[0070]
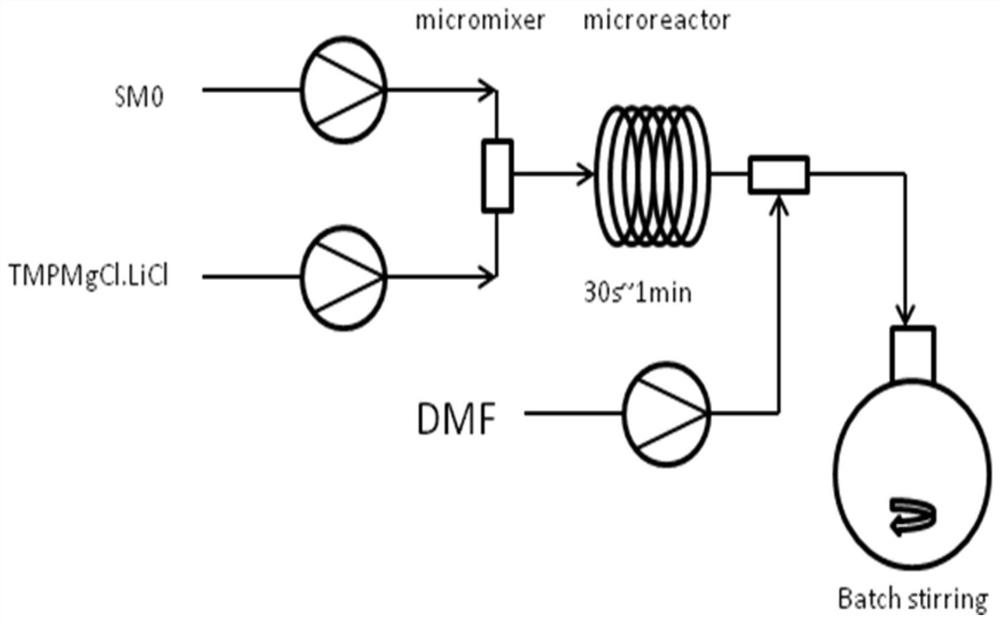
[0071] (1) Transport raw material SM through plunger pump 1 with a flow rate of 6mL / min as Feed 1; TMPMgCl.LiCl is dissolved in THF solution with a concentration of 1mol / L and transported through plunger pump 2 with a flow rate of 69.6mL / min , as Feed 2; build fixtures such as figure 1 As shown, set the molar ratio of Feed 1 and 2 to 1:1.1, set the temperature to 0-20°C, turn on the injection pump, the reaction starts, and the residence time is 30s to 1min; the DMF is transported through the plunger pump 3 with a flow rate of 7.3 mL / min, as Feed 3; mix with Feed 3 at the outlet of the microchannel; increase mechanical stirring in the reaction bottle; after the reaction, pour into ice water to quench, add 2N dilute hydrochloric acid to adjust the pH value to 8; add diatomaceous earth , stirred ev...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com