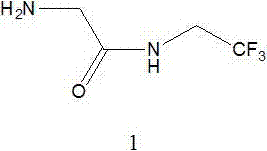
Method for preparing 2-amino-N-(2,2,2-trifluoroethyl)acetamide
A technology of trifluoroethyl and trifluoroethylamine, which is applied in the field of preparing 2-amino-N-acetamide and its salts, can solve the problems of harsh conditions, intrinsic safety, high cost, and expensive catalyst, and achieves the reaction conditions Gentle, Inexpensive, Simple Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
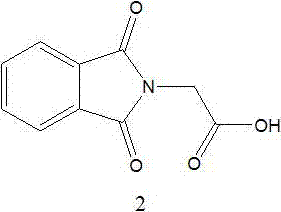
[0035] 1) 2-(1,3-Dioxo-1,3-dihydro-isoindol-2-yl)-N-(2,2,2-trifluoroethyl)-acetamide (Compound 4) preparation of
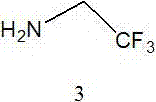
[0036] Dissolve phthalylglycine (5.00 g, 0.024 mol) and DMAP (0.44 g, 0.0036 mol) in 200ml of dichloromethane, cool down to below 0°C, add DCC (5.59 g, 0.0288 mol), and at 0°C After stirring for 30 minutes, trifluoroethylamine hydrochloride (3.52 g, 0.026 mol) and triethylamine (6.7 ml, 0.048 mol) were added, and the reaction was continued at room temperature for 12 hours. Cool down to below 0°C, remove the white precipitate by filtration, wash the filtrate with 200ml of water, separate the organic phase, dry over anhydrous magnesium sulfate, filter, concentrate and crystallize to obtain 4.25g of compound 4 (yield 60.9%). 1 H NMR (DMSO- d6 ) : 8.94(tr, J =6.0Hz, 1H), 7.94-7.88(m, 4H), 4.29(s, 2H), 3.99-3.90(m, 2H).
[0037] 2) Preparation of 2-amino-N-(2,2,2-trifluoroethyl)acetamide (compound 1)
[0038] Add compound 4 (4 g, 0.014 mol), 100 ml of ethanol and ...
Embodiment 2
[0041] 1) 2-(1,3-Dioxo-1,3-dihydro-isoindol-2-yl)-N-(2,2,2-trifluoroethyl)-acetamide (Compound 4) preparation of
[0042]Dissolve phthalylglycine (5.00 g, 0.024 mol) in 100ml N,N-dimethylformamide, add diisopropylethylamine (12.5ml, 0.072mol) and HATU (13.69g, 0.036mol) After stirring and reacting at room temperature for 1 hour, trifluoroethylamine (2.38 g, 0.024 mol) was added, and the reaction was continued at room temperature for 18 hours. The solvent was removed by rotary evaporation, 100ml of ethyl acetate was added and stirred for 30 minutes, washed with 300ml of water, the organic phase was separated, dried over anhydrous magnesium sulfate, filtered, concentrated and crystallized to obtain 5.12g of compound 4 (yield 73.4%).
[0043] 2) Preparation of 2-amino-N-(2,2,2-trifluoroethyl)acetamide
[0044] Add compound 4 (4.58 g, 0.016 mol), 160 ml of ethanol and 40% hydrazine hydrate (4.0 ml, 0.032 mol) into the reaction flask, stir at 40°C for 8 hours, filter to remove th...
Embodiment 3
[0047] 1) 2-(1,3-Dioxo-1,3-dihydro-isoindol-2-yl)-N-(2,2,2-trifluoroethyl)-acetamide (Compound 4) preparation of
[0048] Dissolve phthalylglycine (5.00 g, 0.024 mol) in 200ml ethyl acetate, add CDI (3.89 g, 0.024 mol), stir at room temperature for 1 hour, then add triethylamine (5.0 ml, 0.036mol ) and trifluoroethylamine hydrochloride (3.52 g, 0.026 mol), continue to react at room temperature for 12 hours. The reaction was quenched with 50ml of 1N hydrochloric acid, the organic phase was separated, washed with 200ml of water, dried over anhydrous magnesium sulfate, filtered, and the solvent was removed by rotary evaporation to obtain 5.13g of compound 4 (yield 73.5%).
[0049] 2) Preparation of 2-amino-N-(2,2,2-trifluoroethyl)acetamide (compound 1)
[0050] Add compound 4 (4.58 g, 0.016 mol), 60 ml of methanol and 80% hydrazine hydrate (1.3 ml, 0.021 mol) into the reaction flask, stir at room temperature for 24 hours, filter to remove the white precipitate, and spin evapora...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com