A method for trifluoroethylation of aromatic secondary amines catalyzed by iron porphyrin
A technology of iron porphyrin catalyzing aromatic secondary amine trifluoroethyl, aromatic secondary amine, applied in chemical instruments and methods, catalytic reaction, physical/chemical process catalyst, etc., can solve the problem of high reaction temperature, inapplicability, substrate functional group Poor tolerance and other problems, to achieve the effect of simple operation, low cost, and few reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Synthesis of N-methyl-N-(2,2,2-trifluoroethyl)aniline
[0048] Add 2mmol trifluoroethylamine hydrochloride, 1mL water, 34uL acetic acid, 1mL dichloroethane into the reaction tube, cover with a rubber stopper, and fix it on the stirrer. Take 42mg of sodium nitrite in a 1.5mL sample tube, add 1mL of water to the sample tube, shake the sample tube to dissolve the sodium nitrite. Add the dissolved sodium nitrite solution dropwise into the reaction tube with a syringe, and keep stirring for half an hour at room temperature. Dissolve TPPFeCl (catalytic amount, 9 / 1000 of the molar amount of secondary amine) with 1 mL of dichloroethane, and take 0.24 mmol of N-methylaniline in the sample tube. Half an hour later, the mixed solution in the sample tube was added dropwise into the reaction tube, stirred while adding, and the temperature was raised to 80°C for 12 hours. The reaction solution was cooled to room temperature, filtered to remove some impurities, concentrated and puri...
Embodiment 2
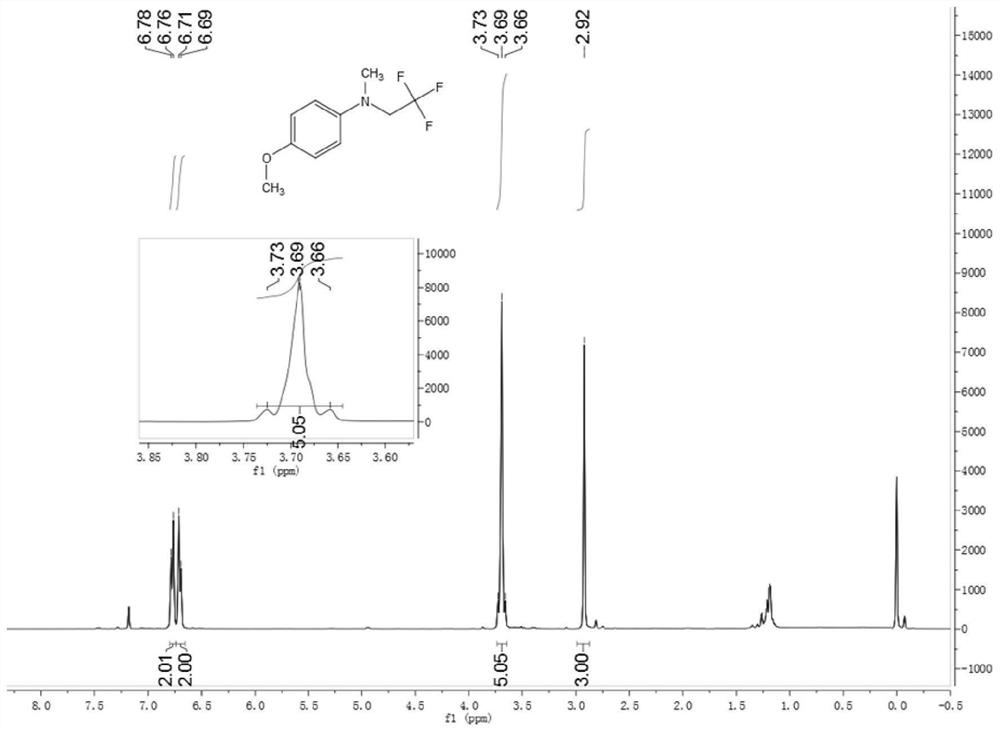
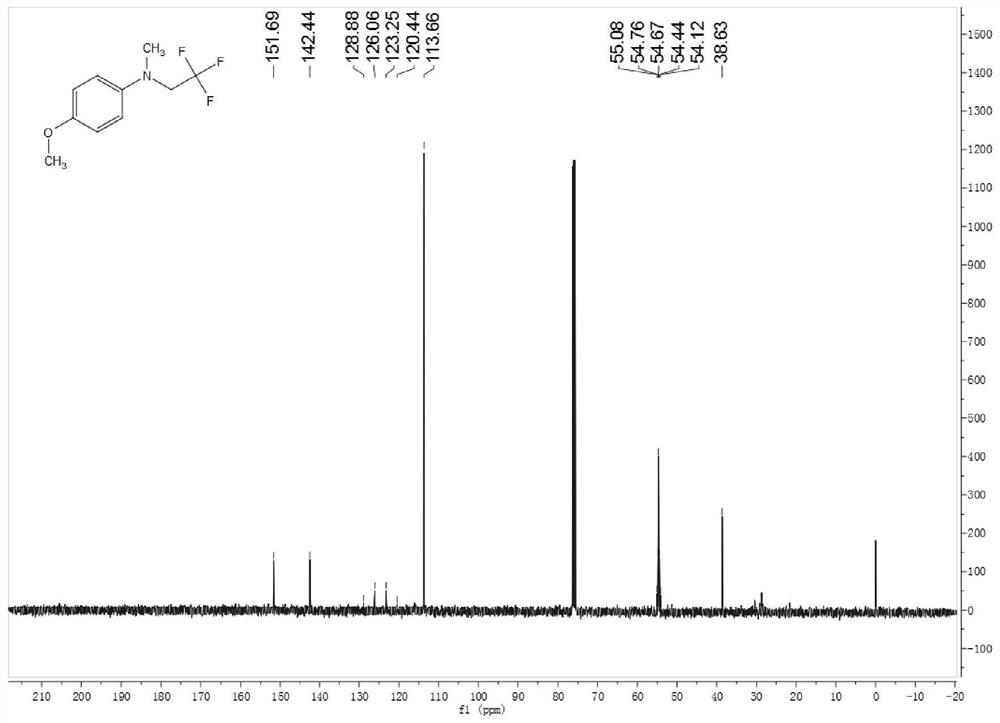
[0086] Synthesis of 4-methoxy-N-methyl-N-(2,2,2-trifluoroethyl)aniline
[0087] Add 2mmol trifluoroethylamine hydrochloride, 1mL water, 34uL acetic acid, 1mL dichloroethane into the reaction tube, cover with a rubber stopper, and fix it on the stirrer. Take 42mg of sodium nitrite in a 1.5mL sample tube, add 1mL of water to the sample tube, shake the sample tube to dissolve the sodium nitrite. Add the dissolved sodium nitrite solution dropwise into the reaction tube with a syringe, and keep stirring for half an hour at room temperature. Dissolve the iron porphyrin (R 2 =R 3 = H, R 1 =Cl, L=Cl) (catalytic amount, 9 / 1000 of the molar amount of secondary amine), take 0.24 mmol of 4-methoxy-N-methylaniline in a sample tube. Half an hour later, the mixed solution in the sample tube was added dropwise into the reaction tube, stirred while adding, and the temperature was raised to 80°C for 12 hours of reaction. The reaction solution was cooled to room temperature, filtered to rem...
Embodiment 3
[0092] Synthesis of N,4-Dimethyl-N-(2,2,2-trifluoroethyl)aniline
[0093] Add 2mmol trifluoroethylamine hydrochloride, 1mL water, 34uL acetic acid, 1mL dichloroethane into the reaction tube, cover with a rubber stopper, and fix it on the stirrer. Take 42mg of sodium nitrite in a 1.5mL sample tube, add 1mL of water to the sample tube, shake the sample tube to dissolve the sodium nitrite. Add the dissolved sodium nitrite solution dropwise into the reaction tube with a syringe, and keep stirring for half an hour at room temperature. Dissolve the iron porphyrin (R 1 = H, R 2 =R 3 = CF 3 , L=OAc) (catalytic amount, 9 / 1000 of the molar amount of secondary amine), take 0.24 mmol of N,4-dimethylaniline in a sample tube. Half an hour later, the mixed solution in the sample tube was added dropwise into the reaction tube, stirred while adding, and the temperature was raised to 80°C for 12 hours of reaction. The reaction solution was cooled to room temperature, filtered to remove so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com