Method for analyzing related impurities of isoxazoline veterinary drug intermediate ammonium salt
An analytical method and isoxazoline technology, which can be used in analytical materials, material separation, instruments, etc., can solve many problems, and achieve the effect of good separation effect and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] 1) Preparation of standard reference substance solution: Accurately weigh 2-amino-N-(2,2,2-trifluoroethyl)acetamide standard substance (hereinafter referred to as AY standard substance) and add diluent (the diluent is every 1ml Containing 20 μg of ascorbic acid solution, the same below) dissolved, and prepared into a reference substance solution containing 15 mg of working standard per milliliter of AY standard substance per 1 mL.
[0044] 2) Preparation of the test solution: Accurately weigh 2-amino-N-(2,2,2-trifluoroethyl)acetamide for the test, add diluent and dissolve to make 75 μg of the test in 1ml The solution.
[0045] 3) Impurity D stock solution: Accurately weigh 10 mg of the impurity D reference substance, put it in a 10 ml volumetric flask, add diluent to dissolve and set the volume to the mark, and make a stock solution containing 1 mg of impurity D in each 1 ml.
[0046] 4) Instrument: high performance liquid chromatography (CAD detector);
[0047] Chrom...
Embodiment 2
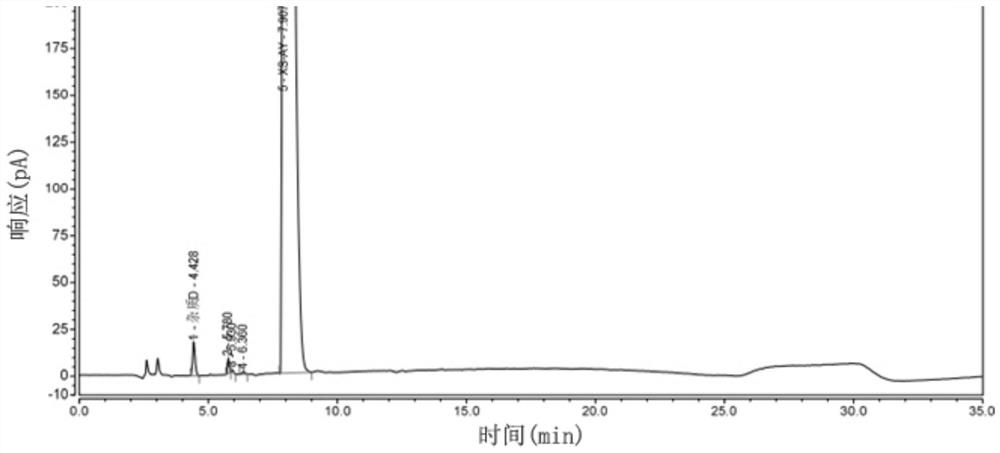
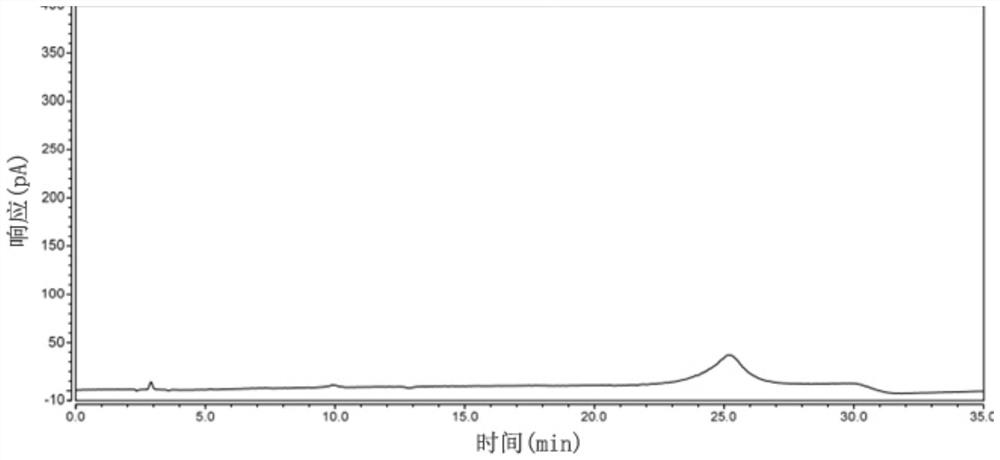
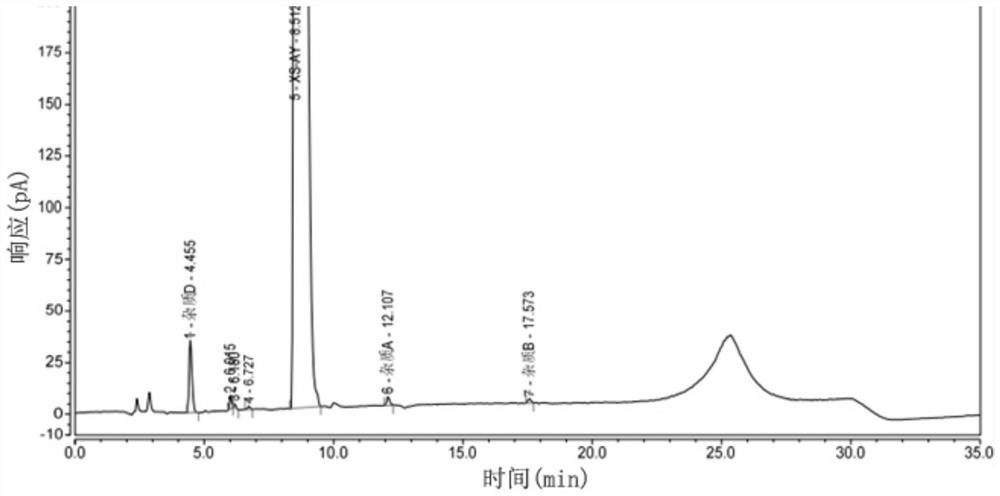
[0058] Example 2: Specificity
[0059] Prepare reference substance solution and need testing solution and impurity D stock solution according to embodiment 1;
[0060] Preparation of system suitability solution: prepare a system suitability solution containing 15mg of the test product and 75μg each of impurity A, impurity B, and impurity D per 1ml;
[0061] Take the test solution to prepare various destruction solutions:
[0062] Light damage solution: take the stock solution of the test product into a transparent EP tube and place it under 254nm ultraviolet light for 24 hours;
[0063] Oxidative destruction solution: take the test product stock solution and add 3% H 2 o 2 , placed at room temperature for 4 hours;
[0064] High-temperature destruction solution: take 1ml of the stock solution of the test product, put it in a transparent EP tube, place it in a water bath at 60°C for 3 hours, and then cool it down;
[0065] Acid destruction solution: take the test product stoc...
Embodiment 3
[0074] Embodiment 3: detection limit and quantitative limit
[0075] 1) Impurity D stock solution: Accurately weigh 10 mg of the impurity D reference substance, put it in a 10ml volumetric flask, add diluent to dissolve and set the volume to the mark, and make a stock solution containing 1mg of impurity D in each 1ml.
[0076] According to the chromatogram of each related substance limit solution in Example 2, the signal-to-noise ratio of each component can be preliminarily obtained, and the stock solution is gradually diluted to find out the concentration corresponding to the signal-to-noise ratio of 2 to 4, which is defined as the detection limit concentration; The concentration corresponding to the signal-to-noise ratio of 8 to 12 was defined as the concentration of quantitation limit. The results are shown in Table 4 and Table 5 below.
[0077] Table 4. Results of detection limit of impurity D reference substance
[0078]
[0079] Table 5, results of quantitation limi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com