Preparation method of trifluoroethylamine
A technology of trifluoroethylamine and difluoroethylamine, which is applied in the field of preparation of trifluoroethylamine, can solve problems such as difficult industrialization, numerous routes, and difficult operation, and achieve low cost, easy cost, and simple reaction operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
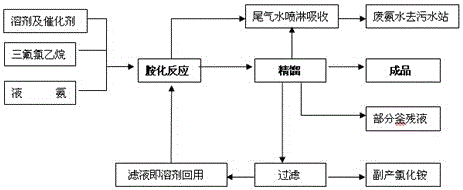
[0024] Such as figure 1 Shown, a kind of preparation method of trifluoroethylamine comprises the steps:
[0025] The first step, amination reaction: with CHF 2 CH 2 X is a raw material, and in the presence of a solvent and a catalyst, it is ammoniated with ammonia gas to generate trifluoroethylamine, and the reaction equation is as follows:
[0026] CHF 2 CH 2 Cl + 2NH 3 → CF 3 CH 2 NH 2 +NH 4 Cl;
[0027] The solvent is: NMP or DMF.
[0028] The specific operation of the amination reaction is as follows: using a batch process, add solvent, liquid ammonia and an appropriate amount of catalyst in sequence in a high-pressure reactor equipped with an exhaust gas absorption device, start stirring, heat up, and slowly feed CHF at a specified speed at 30°C 2 CH 2 Cl reaction, observe the internal temperature of the kettle. When the temperature of the kettle rises to about 110°C, the pressure of the kettle is at 2.0MPa, and the reaction is kept for 16 hours. The reactio...
Embodiment 2
[0032] Such as figure 1 Shown, a kind of preparation method of trifluoroethylamine comprises the steps:
[0033] The first step, amination reaction: with CHF 2 CH 2 Br is a raw material, and in the presence of a solvent and a catalyst, ammonia gas is ammoniated to generate trifluoroethylamine, and the reaction equation is as follows:
[0034] CHF 2 CH 2 Br + 2NH 3 → CF 3 CH 2 NH 2 +NH 4 Br;
[0035] The solvent is: DMSO.
[0036] The specific operation of the amination reaction is as follows: using a batch process, add solvent, liquid ammonia and an appropriate amount of catalyst in sequence into a high-pressure reactor equipped with an exhaust gas absorption device, start stirring, heat up, and slowly feed CHF at a specified speed at 100°C 2 CH 2 Br reaction, observe the internal temperature of the kettle. When the temperature of the kettle rises to about 210°C, the pressure of the kettle is at 6.0MPa, and the reaction is kept for 36 hours. The reaction is basic...
Embodiment 3
[0040] Such as figure 1 Shown, a kind of preparation method of trifluoroethylamine comprises the steps:
[0041] The first step, amination reaction: with CHF 2 CH 2 I is a raw material, and in the presence of a solvent and a catalyzer, ammonia gas is ammoniated to generate trifluoroethylamine, and the reaction equation is as follows:
[0042] CHF 2 CH 2 I + 2NH 3 → CF 3 CH 2 NH 2 +NH 4 I;
[0043] The solvent is: alkyl glycol: propylene glycol or ethylene glycol.
[0044] The specific operation of the amination reaction is as follows: using a batch process, add solvent, liquid ammonia and an appropriate amount of catalyst sequentially into a high-pressure reactor equipped with an exhaust gas absorption device, start stirring, heat up, and slowly feed CHF at a specified speed at 80°C 2 CH 2 1 reaction, observe the temperature situation in the kettle, when the temperature of the kettle rises to about 170°C, the pressure of the kettle is at 4.0MPa, and the heat pres...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com