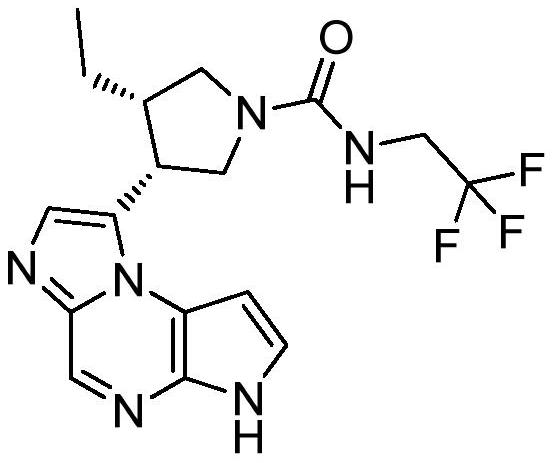
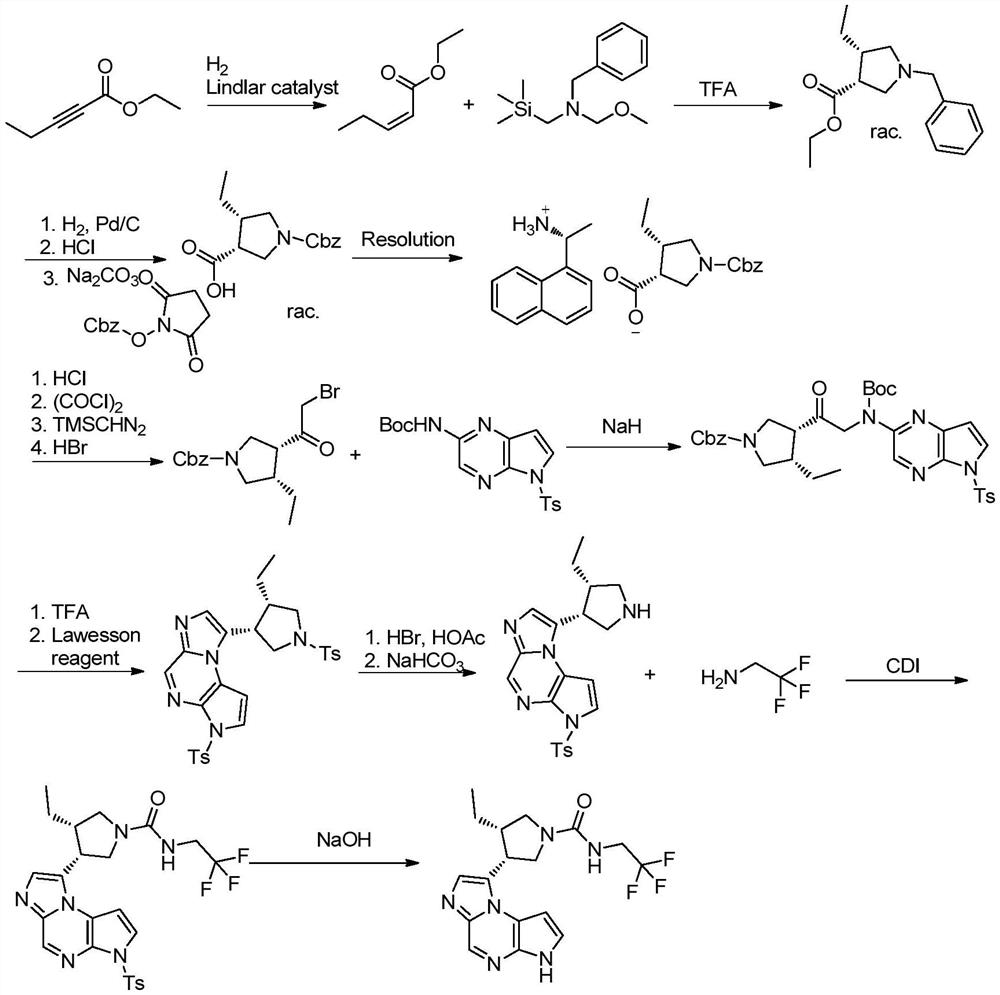
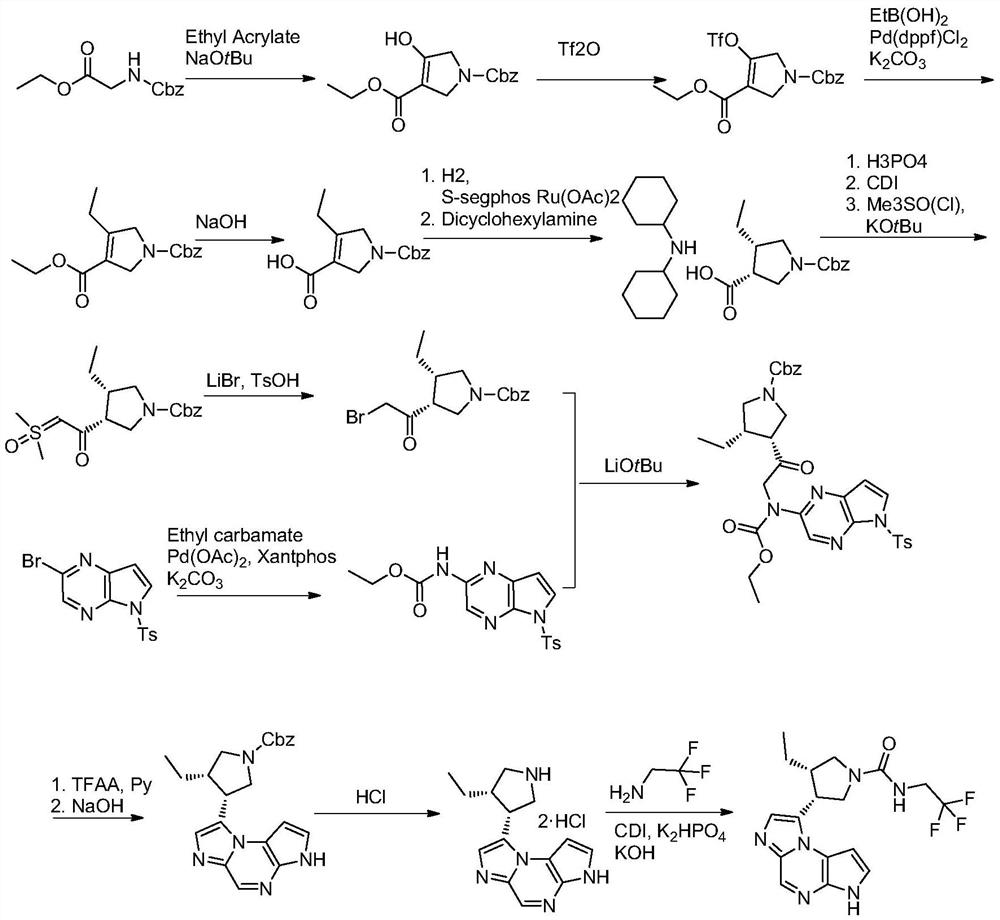
The synthetic method of upadatinib
A synthetic method and salt-forming technology, which is applied in the field of medicine and chemical industry, and can solve the problems of low total yield, long synthesis steps, and high price of process amplification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069]
[0070] Transfer 2-bromo-5-p-toluenesulfonyl-5H-pyrrolo[2,3-b]pyrazine 7 (35.22g, 100mmol) into a sealed reaction flask, add ethylene glycol (105mL), copper oxide (398mg , 5.0mmol), L-proline (1.15g, 10.0mmol), potassium carbonate (27.64g, 200mmol), ammonia water (25%, 105g), after sealing, heat the oil bath to an internal temperature of 110~120℃ for 36~ After 48 hours, the reaction was completed and cooled to room temperature, added 15% brine (140mL), added isopropyl acetate (175mL) for extraction 3 times, combined the organic phases and washed 2 times with saturated brine (105mL), dried over sodium sulfate, concentrated to remove most of the Solvent, add petroleum ether (215mL) for slurry, filter and dry to obtain compound 8 (25.37g, 88%).
[0071] Here copper oxide can be replaced by cuprous iodide, cuprous bromide, cuprous oxide, cupric bromide or cupric chloride; Methylformamide, dimethylacetamide, N-methylpyrrolidone, 1,4-dioxane or toluene instead; L-proline...
Embodiment 2
[0073]
[0074] Add compound formula 8 (28.83g, 100mmol) and dichloromethane (144mL) into the three-necked flask, add triethylamine (20.24g, 200mmol), stir well, cool to 0~5°C, add methanesulfonyl chloride (12.60 g, 110mmol), warming up to room temperature and reacting for 6-8 hours after dropping. After the reaction was completed, saturated ammonium chloride solution (144mL) was added, the organic phase was separated, the aqueous phase was extracted three times with ethyl acetate (144mL), the combined organic phase was washed once with saturated brine (144mL), dried over sodium sulfate, and concentrated Slurry was added with petroleum ether (144 mL), filtered and dried to obtain compound formula 9a (33.34 g, 91%).
[0075] MS(ESI)m / z=367.0[M+H] + , 1 H NMR(400MHz,DMSO-d6)δ11.14(br,1H),8.22(d,J=16.4Hz,2H),8.01(s,2H),7.40(s,2H),6.96(s,1H) ,3.44(s,3H),2.30(s,3H).
[0076] Here triethylamine can be replaced by diisopropylethylamine, DMAP or pyridine; solvent dichloromethan...
Embodiment 3
[0078]
[0079] Add compound formula 8 (28.83g, 100mmol) and dichloromethane (144mL) into the three-necked flask, add diisopropylethylamine (25.85g, 200mmol), stir evenly, cool to 0-5°C, drop p-toluenesulfonate Acyl chloride (20.97g, 110mmol), warm up to room temperature and react for 6-8 hours after dropping. After the reaction was completed, saturated ammonium chloride solution (144mL) was added, the organic phase was separated, the aqueous phase was extracted three times with ethyl acetate (144mL), the combined organic phase was washed once with saturated brine (144mL), dried over sodium sulfate, and concentrated Slurry was added with petroleum ether (144 mL), filtered and dried to obtain compound formula 9b (41.60 g, 94%).
[0080] MS(ESI)m / z=443.1[M+H] + , 1 H NMR (400MHz, DMSO-d6) δ11.45 (br, 1H), 8.20 (d, J = 4.0Hz, 1H), 8.17 (s, 1H), 7.96 (d, J = 8.4Hz, 2H), 7.86 (d,J=8.0Hz,2H),7.40(d,J=8.4Hz,2H),7.34(d,J=8.0Hz,2H),6.85(d,J=4.0Hz,1H),2.31(s ,3H), 2.30(s,3H).
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com