Preparation method of trifluoroisothiocyanate ethane
A technology of isothiocyanate and trifluoroethylamine is applied in the field of preparation of trifluoroisothiocyanate, can solve the problems of unsuitability for industrial production, low actual yield, low yield and the like, and achieves low toxicity, The effect of improving reaction yield and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
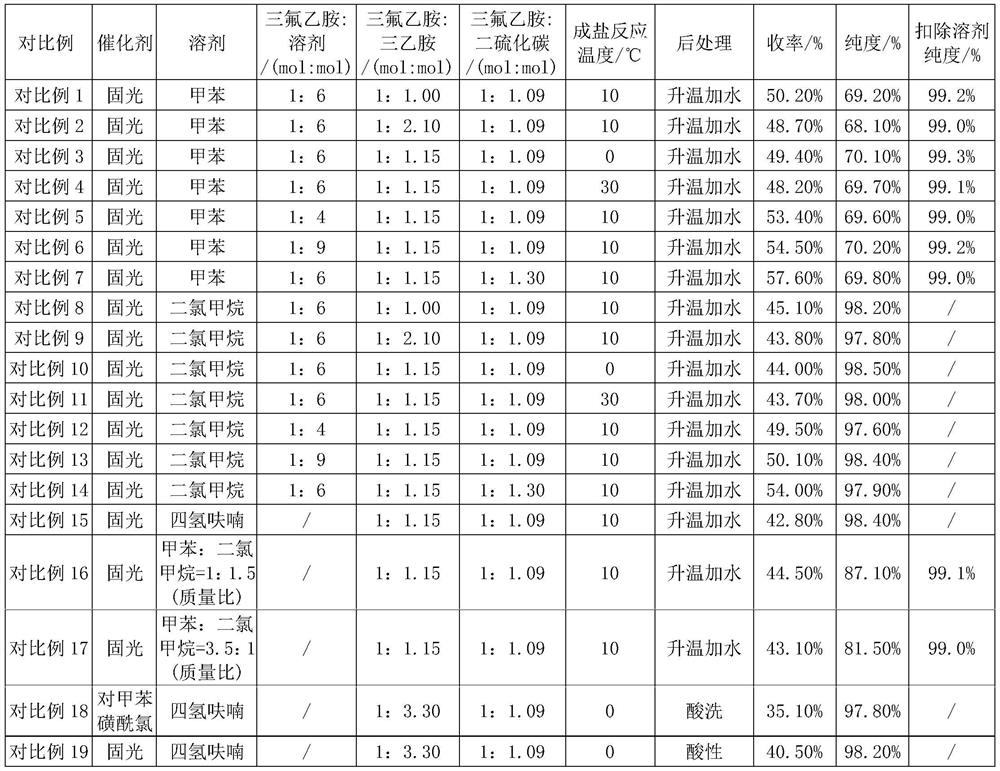
Examples
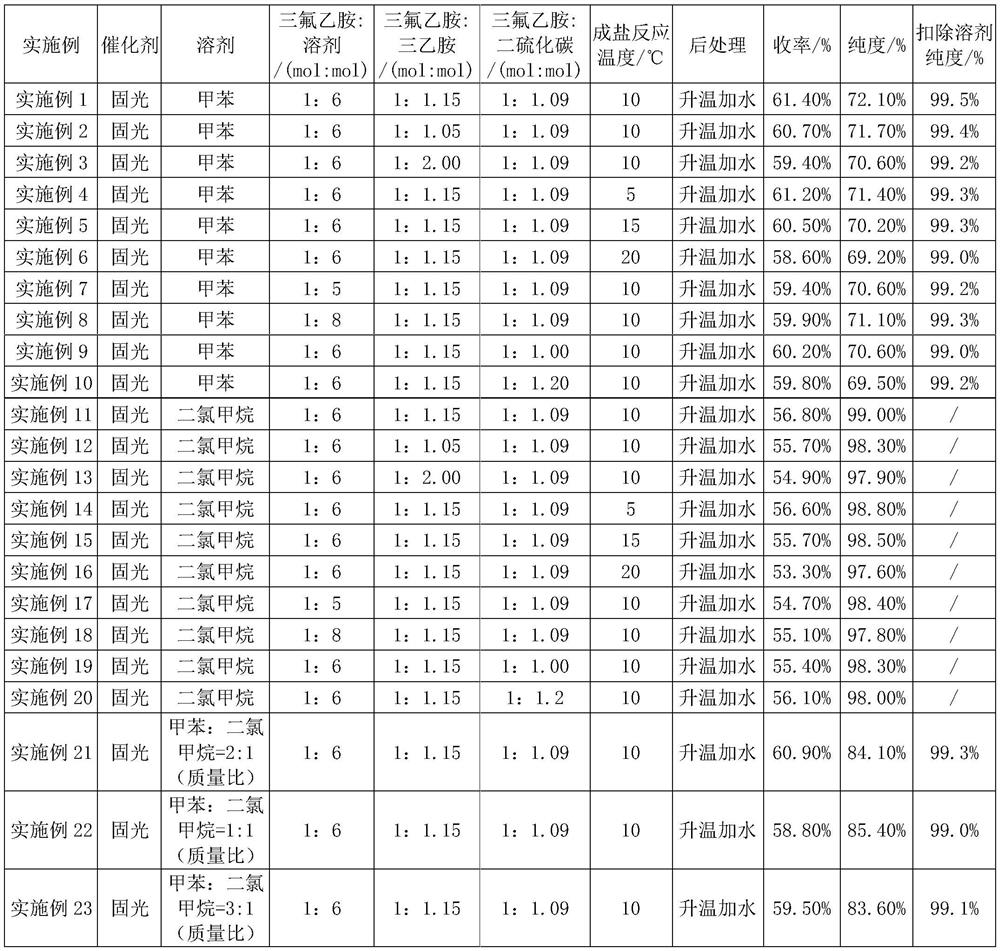
Embodiment 1
[0020] Embodiment 1: drop into 40.0g trifluoroethylamine (0.404mol, 1eq), 47.0g triethylamine (0.465mol, 1.15eq) and 130.0g toluene (1.411mol) in the round bottom bottle, stir and cool to 10-12 ℃, slowly add 33.5g of carbon disulfide (0.440mol, 1.09eq) dropwise, after the dropwise completion, react at a temperature of 10℃ for 10 hours to obtain trifluoroethylaminothioformate. Then lower the temperature to 5-10°C, slowly add 139.0g of solid light-toluene mixed solution (containing 0.154mol of solid light, 0.38eq; toluene 1.013mol) dropwise, and stir at room temperature for 2 hours, then heat up to 40-45°C (Act to remove fixed light) react for 3 hours. After the sampling test is qualified (trifluoroethylamine content ≤ 0.5%), the temperature of the reaction solution is lowered to 5-10°C, water is added, stirred, allowed to stand for stratification, and the organic phase is washed with water, dried and rectified to obtain trifluoroisothiocyanate Ethane toluene liquid. used dire...
Embodiment 2
[0022] Embodiment 2: except that the consumption of triethylamine is replaced by 42.9g (1.05eq), all the other are the same as embodiment 1.
[0023] The product yield is 60.7%; the purity is 71.7%, and the purity after deducting toluene is 99.4%.
Embodiment 3
[0024] Embodiment 3: except that triethylamine consumption is replaced by 81.8g (2.0eq), all the other are the same as embodiment 1.
[0025] The yield of the product is 59.4%, the purity is 70.6%, and the purity after deducting toluene is 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com