Fatty trifluoroethyl ester compound and its preparation method
A technology for trifluoroethyl ester and compound is applied in the field of aliphatic trifluoroethyl ester compound and its preparation, which can solve the problems of poor atom utilization, low reaction efficiency, harsh reaction conditions and the like, achieves less side reactions and is beneficial to environmental protection , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
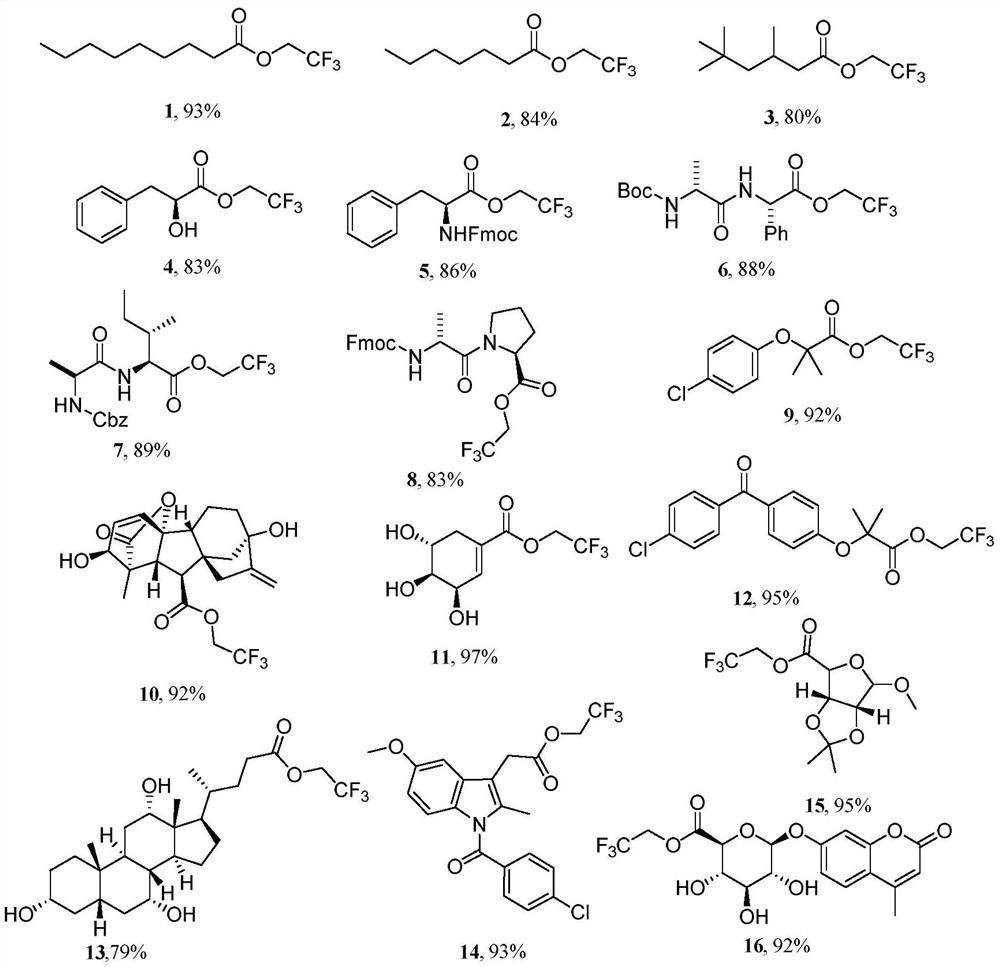
Embodiment 1~16
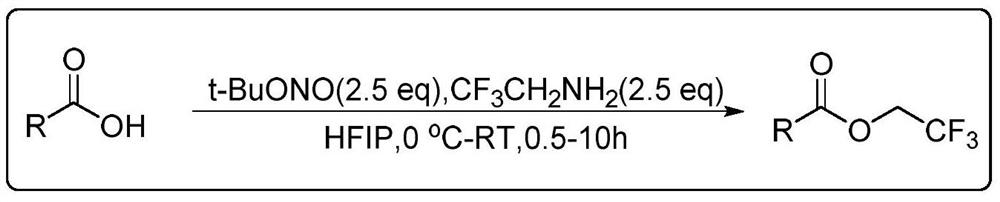
[0027] The following implementations 1-16 are prepared according to the following synthetic route, and compounds 1-16 can be prepared:
[0028]
[0029] The specific operation is: dissolve the fatty acid (0.5mmol, 1equiv) substrate in 1,1,1,3,3,3-hexafluoro-2-propanol, and add tert-butyl nitrite ( 150μL, 1.25mmol, 2.5equiv) and 2,2,2-trifluoroethylamine (100μL, 1.25mmol, 2.5equiv), stirred for 5min and raised to room temperature to continue the reaction for 0.5~10h, and the solution in the reaction system was decompressed After spinning to dryness, the residue was purified by column chromatography to obtain fatty trifluoroethyl ester compounds.
Embodiment 1
[0031] Substrate: nonanoic acid
[0032] product:
[0033] Compound 1: colorless oil (107mg, 93% yield); IR(KBr)ν max / cm -1 :2959,2930,2859,1762, 1457,1412,1283,1170,1111,1067,979,842,807; 1 H NMR (400MHz, CDCl 3 )δ4.39(q, J=8.5 Hz, 2H, C H 2 CF 3 ), 2.34(t, J=7.5Hz, 2H, COC H 2 ),1.72–1.48(m,2H,COCH 2 C H 2 ), 1.22 (dd, J=12.8, 6.6Hz, 10H, C H 2 ),0.81(t,J=6.8Hz,3H,CH 2 C H 3 );13 C NMR (100MHz, CDCl 3 )δ 171.2(CO), 122.0(q, J=277.1Hz, C f 3 ),59.1(q,J=36.5Hz, C CF 3 ), 32.6 (CO C h 2 ), 30.8 (COCH 2 C h 2 ), 28.13, 28.08, 28.0, 23.7, 21.6, 13.0 (CH 2 C h 3 ); 19 F NMR (376MHz, CDCl 3 )δ -73.97(t, J=8.3Hz, C F 3 ); 19 F{ 1 H}NMR (376MHz, CDCl 3 )δ-73.97(s,C F 3 ); HRMS(ESI): m / zcalcd for C 11 h 29 f 3 o 2 Na + [M+Na] + 263.1229, found 263.1242.
Embodiment 2
[0035] Substrate: Heptanoic acid
[0036] product:
[0037] Compound 2: colorless oil (89mg, 84% yield); IR(KBr)ν max / cm -1 :2961,2931,2858,1756,1419, 1285,1172,1107,909,807,735; 1 H NMR (400MHz, CDCl 3 )δ4.47(q, J=8.5Hz, 2H, C H 2 CF 3 ), 2.42(t,J=7.5Hz,2H,COC H 2 ), 1.66 (p, J=7.5Hz, 2H, C H 2 CH 3 ),1.48–1.22(m,6H),0.88(t,J=6.9Hz,3H,C H 3 ); 13 C NMR (100MHz, CDCl 3 )δ172.2(CO), 123.0(q, J=277.1Hz, C h 2 CF 3 ), 60.1(q, J=36.4Hz, C f 3 ), 33.7 (CO C h 2 ),31.4( C h 2 ),28.7( C h 2 ),24.7( C h 2 ),22.5( C h 2 ),14.0 ( C h 3 ); 19 F NMR (376MHz, CDCl 3 )δ-73.85(t, J=8.5Hz, C F 3 ); 19 F{ 1 H}NMR (376MHz, CDCl 3 ) δ-73.85(s,C F 3 ); HRMS(ESI):m / z calcd for C 9 h 15 f 3 o 2 Na + [M+Na] + 235.0916,found235.0916.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com