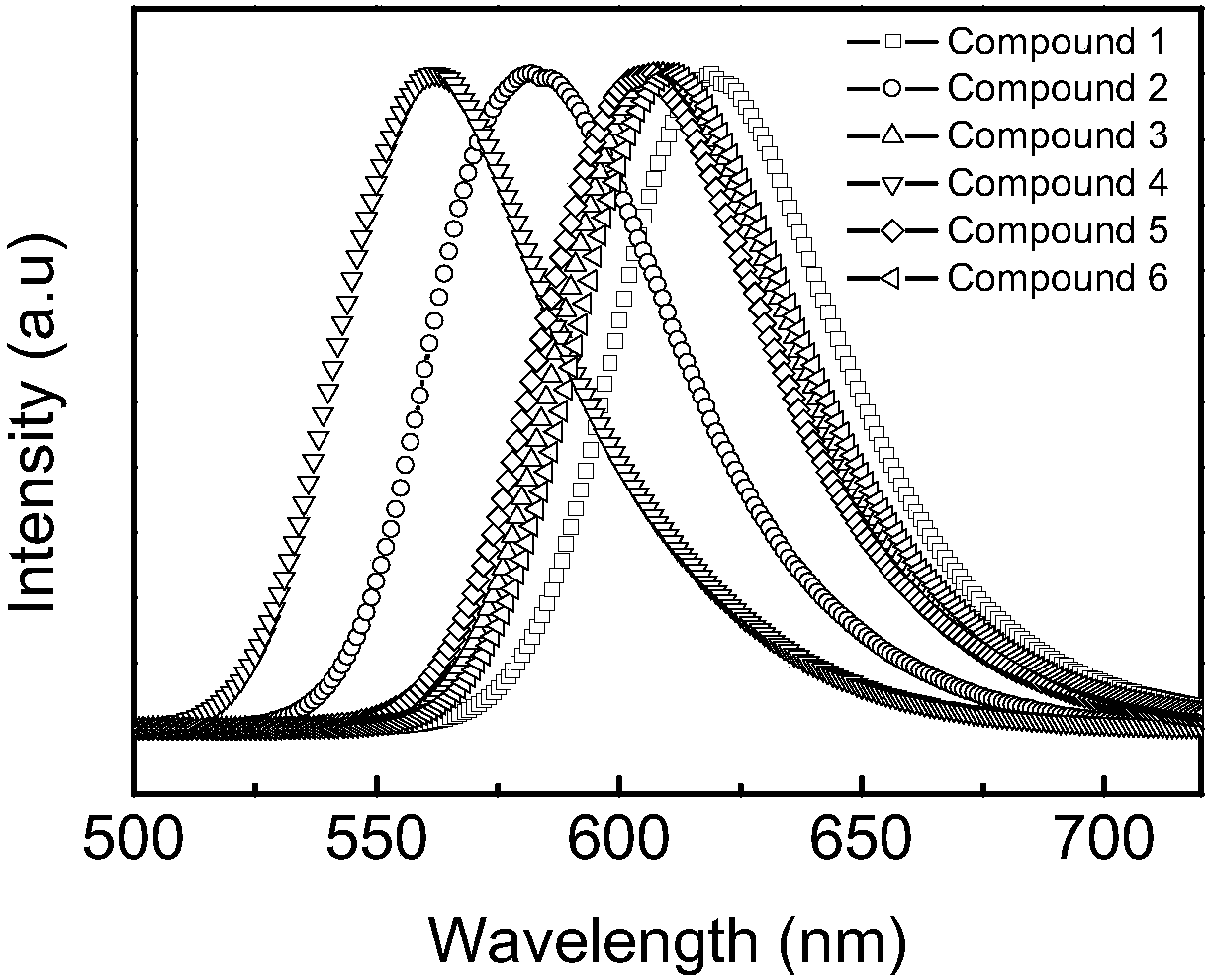
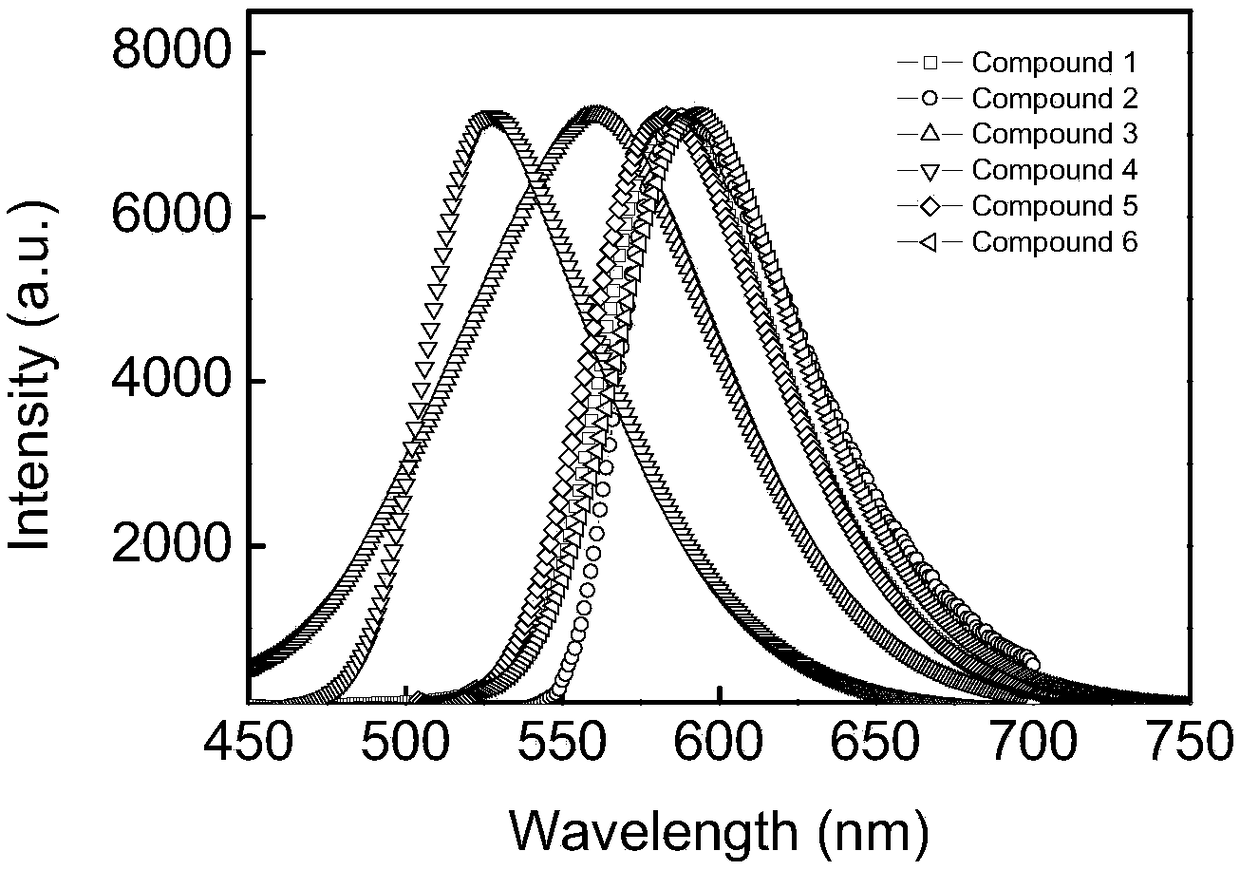
Method for 2, 5-dimethoxyacyl-1, 4-cyclohexanedione amination by reaction kettle
A technology based on cyclohexanedione amine and dimethoxyacyl, which is applied in the field of organic fluorescent materials, can solve the problems of fluorescence quenching, unfavorable fluorescence emission, weak fluorescence, etc., achieve cost saving, avoid reflux condensation device, and simple synthesis method Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] a. Add 658mg of 2,5-dimethoxyacyl-1,4-cyclohexanedione and 2.46mL of 30% methylamine methanol solution in 10mL of methanol as solvent in the reaction kettle;
[0024] b. Put it in an oven at 150°C and react for 8 hours;
[0025] c. Suction filtration with Buchner funnel, rinse and dry with methanol to obtain 455 mg of product, yield 60%.
[0026] The obtained compound 1, 1 H NMR (400MHz, CDCl 3 ): δ8.84(s, 2H), 3.74(s, 6H), 3.24(s, 4H), 2.96(d, J=5.2Hz, 6H). 13 C NMR (400MHz, CDCl 3 ): δ169.8, 159.0, 83.3, 50.4, 29.2, 26.6.
Embodiment 2
[0028] a. Add 658mg of 2,5-dimethoxyacyl-1,4-cyclohexanedione and 2.74mL of aniline to the reaction kettle with 10mL of methanol as the solvent;
[0029] b. Put it in an oven at 150°C and react for 8 hours;
[0030] c. Suction filtration with Buchner funnel, rinse and dry with methanol to obtain 633 mg of product, yield 56%.
[0031] The obtained compound 2, 1 H NMR (400MHz, CDCl 3 ): δ10.7(s,2H),7.1-7.4(m,10H),3.68(s,6H),3.42(s,4H). 13 C NMR (400MHz, CDCl 3 ): δ169.6, 157.0, 139.4, 129.3, 124.9, 89.0, 51.0, 28.0.
Embodiment 3
[0033] a. Add 658mg of 2,5-dimethoxyacyl-1,4-cyclohexanedione and 2.61mL of 2-methoxyethylamine to the reaction kettle with 10mL of methanol as solvent;
[0034] b. Put it in an oven at 150°C and react for 8 hours;
[0035] c. Suction filtration with Buchner funnel, rinse and dry with methanol to obtain 590mg of the product with a yield of 57%.
[0036]The obtained compound 3, 1H NMR, 400MHz, CDCl3: δ9.03(s, 2H), 3.71(s, 6H), 3.55(t, 4H), 3.47(q, 4H), 3.42(s, 6H), 3.24( s, 4H); 13C NMR, 400MHz, CDCl3: δ169.7, 157.6, 84.1, 71.8, 59.1, 50.5, 42.3, 26.9.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com