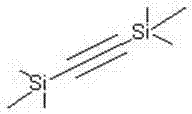
Synthetic method of di(trimethyl silicon-based)acetylene
A technology of bistrimethylsilylacetylene and bistrimethylsilyl, which is applied in the field of synthesis of bistrimethylsilylacetylene, which can solve the problems of high cost, difficulty in recovering tetrahydrofuran, and high market price of bistrimethylsilylacetylene and other issues to achieve the effects of reducing production and emissions, reducing synthesis costs, and cheap prices
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] In a 1000 ml three-necked flask, add 350 ml of toluene, add 70 g of disodium acetylene under the protection of nitrogen, cool to 0~10°C, and slowly add 310g of trimethylbromosilane dropwise under the control of 0~10°C, the dropwise addition is completed , raise the temperature to 50~60°C for 2 hours, after the reaction is completed, cool down to 20~30°C, slowly add 200 ml of water dropwise, after dropping, separate layers, extract the water layer twice with 50ml toluene, combine the toluene layers, and use anhydrous Drying over sodium sulfate, concentrating toluene at a vacuum of 40~60mmHg, and then rectifying with a 2*15cm rectification column at a vacuum of 10~20mmHg to obtain more than 99% of the target product bistrimethylsilylacetylene 158g, mol Yield 92.0%.
[0019] The molar mass ratio of acetylene dimetallic salt to trimethylhalosilane is 1:2.0~1:2.2,
Embodiment 2
[0021] In a 500 ml three-necked flask, add 175ml of toluene, add 35g of disodium acetylene under the protection of nitrogen, cool to 0-10°C, and slowly add 200g of iodotrimethylsilane dropwise under the control of 0-10°C. Raise the temperature to 50-60°C and react for 2 hours, after the reaction is complete, cool down to 20-30°C, slowly add 100 ml of water dropwise, after the drop is completed, separate layers, extract the water layer twice with 25ml toluene, combine the toluene layers, and wash with anhydrous sulfuric acid Sodium drying, concentrated toluene at a vacuum of 40~60mmHg, and then rectifying with a 2*15cm rectification column at a vacuum of 10~20mmHg to obtain more than 99% of the target product bistrimethylsilylacetylene 78g, molar yield The rate is 90.8%.
Embodiment 3
[0023] In a 5000 ml three-necked flask, add 1750 ml of toluene, add 350 g of disodium acetylene under the protection of nitrogen, cool to 0~10°C, and slowly add 1100g of trimethylchlorosilane dropwise under the control of 0~10°C, the dropwise addition is completed , heated up to 50~60°C and reacted for 2 hours. After the reaction was completed, lower the temperature to 20~30°C, slowly add 1000 ml of water dropwise. Sodium sulfate drying, under vacuum degree 40~60mmHg, concentrate toluene, then under vacuum degree 10~20mmHg, rectify with 2*15cm rectification column, obtain 99% target product of the present invention bistrimethylsilyl acetylene 802g, The molar yield is 93.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com