Preparation method of intermediate of medicine for treating chronic dry eye
An intermediate and technology for dry eye syndrome, which is applied in the preparation of dry eye drug ritazast intermediate, in the field of dry eye drug intermediates, can solve problems such as high cost, polyphosphorous by-products, and serious environmental pollution. Achieving low cost, high yield and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
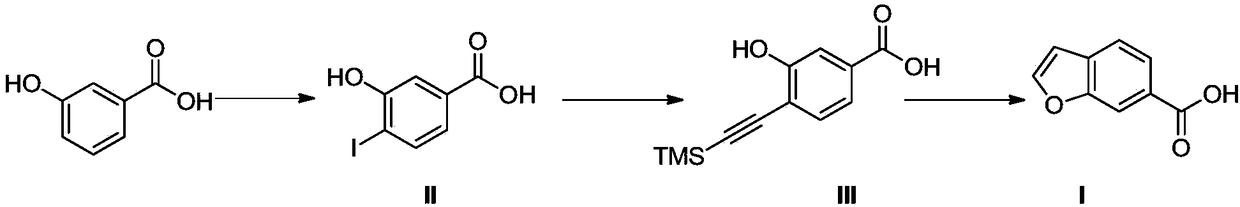
[0014] The preparation method of the dry eye medicine intermediate of the present invention, such as figure 1 As shown, including the following steps:
[0015] (a) Add 2L of methanol, 200g of meta-hydroxybenzoic acid, 61g of NaOH and 226g of NaI to a 5L three-necked flask, add 1.43L of NaClO aqueous solution (mass content of 9%, dropping rate of 2L / h) and control the temperature to -10 ℃~-5℃, react for 2h; Concentrate under reduced pressure at 50℃ to remove methanol, then adjust pH to about 1 with 1N (i.e. equivalent concentration) hydrochloric acid. At this time, a large amount of white solid precipitates, filtered and dried to obtain 280g of compound II; The NMR spectrum of compound II is resolved as: 1 H NMR(400MHz, DMSO-d 6 )δ(ppm): 13.00 (brs, 1H), 10.67 (brs, 1H), 7.78 (d, J = 8.0 Hz, 1H), 7.40 (brs, 1H), 7.11 (d, J = 8.0 Hz, 1H) . ESI-MS m / z calcd:C 7 H 5 IO 3 ([M-H] - ); 262.93, found: 262.9.
[0016] (b) Add 1L of THF (ie tetrahydrofuran) to a 3L three-necked flask, and...
Embodiment 2
[0019] This example provides a method for preparing a dry eye syndrome drug intermediate, which is basically the same as that in Example 1, except that in step (c), 2.2 g of cuprous iodide and 22 g of triethylamine are used Finally, 37.2 g of a yellow solid compound (purity 99.3%) was obtained.
Embodiment 3
[0021] This embodiment provides a method for preparing a dry eye syndrome drug intermediate, which is basically the same as that in Example 1, except that: in step (c), 1.8g cuprous iodide and 18g triethylamine are used Finally, 36.5 g of a yellow solid compound (purity 99.2%) was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com