Triazole-functionalized 4,4'-difluorodiphenylsulfone compounds and synthesis method thereof
A technology of difluorodiphenylsulfone and compounds, which is applied in the field of triazole-functionalized 4,4'-difluorodiphenylsulfone compounds and their synthesis, which can solve the problem of proton conductivity being affected by water content and conductivity decline, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
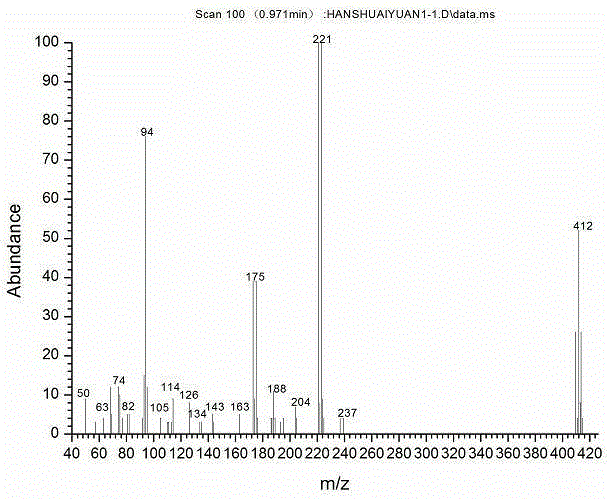
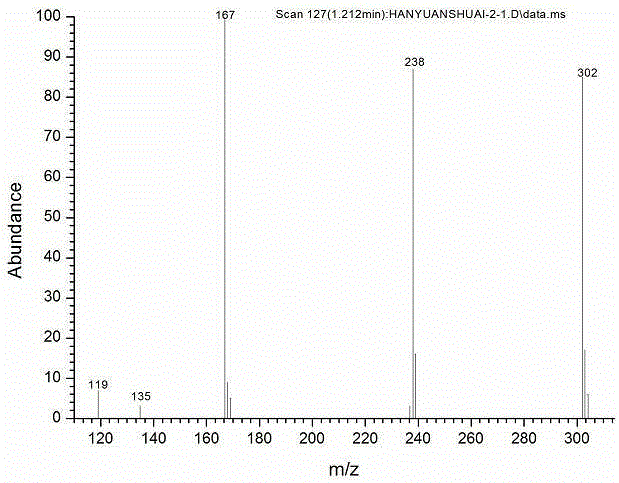
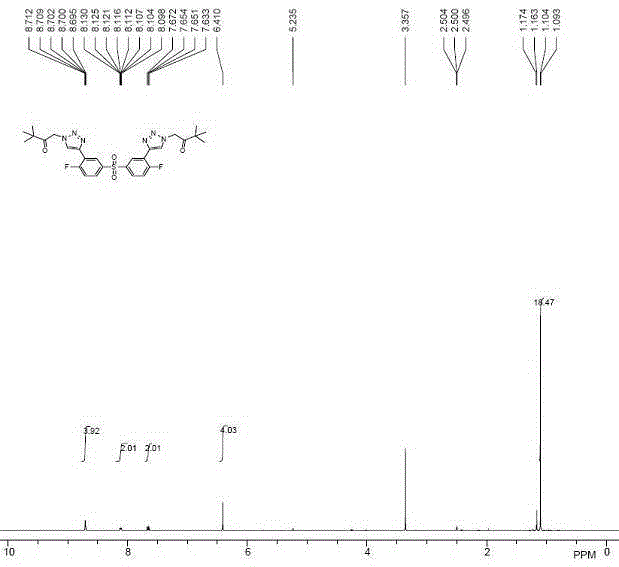
Image
Examples
Embodiment 1
[0032] In this example, 4,4'-difluorodiphenylsulfone is used as raw material to synthesize the target product through bromination, coupling, click and other reactions. The first step is 3,3'-dibromo-4,4'- Synthesis of difluorodiphenyl sulfone, step (2) is 3,3'-bistrimethylsilylethynyl-4,4'-difluorodiphenyl sulfone synthesis, step (3) is 3,3' -Synthesis of diethynyl-4,4'-difluorodiphenylsulfone, step (4) is the synthesis of triazole-functionalized 4,4'-difluorodiphenylsulfone.
[0033] Experimental steps:
[0034] (1) Add 18.32g of 4,4'-difluorodiphenyl sulfone and 45ml of concentrated H to a 50ml round bottom flask 2 SO 4 , stirred at room temperature to completely dissolve 4,4'-difluorodiphenyl sulfone, and then add 28.26g NBS to the above solution. Spot the plate to track the reaction process, after about 6 hours, the reaction is over, the solution after the reaction is poured into a beaker filled with 350 g of ice water, and a yellow-white turbid mixture is obtained afte...
Embodiment 2
[0039] (1) Synthesis of 3,3'-dibromo-4,4'-difluorodiphenylsulfone: Same as Example 1
[0040] (2) Synthesis of 3,3'-bistrimethylsilylethynyl-4,4'-difluorodiphenyl sulfone: same as Example 1
[0041] (3) Dissolve 1.2g of 3,3'-bistrimethylsilylethynyl-4,4'-difluorodiphenyl sulfone in 15ml of THF, then add 1.508g of KOH to the solution, stir the reaction at room temperature, and the reaction is complete Then add 50ml ether and 15ml water, and carry out liquid separation operation. An appropriate amount of anhydrous Na was added to the organic phase 2 SO 4 Dry it. After drying, filter, add a certain amount of silica gel to the filtrate, rotate at 40°C, and separate by column chromatography to obtain 3,3'-ditrimethylsilylethynyl-4,4'-difluorodiphenyl sulfone, which is poured through the column The washing solution is petroleum ether:dichloromethane=8:5, and the yield is 40.8%.
[0042] (4) Synthesis of triazole-functionalized 4,4'-difluorodiphenylsulfone: same as Example 1
Embodiment 3
[0044] (1) Synthesis of 3,3'-dibromo-4,4'-difluorodiphenylsulfone: Same as Example 1
[0045] (2) Synthesis of 3,3'-bistrimethylsilylethynyl-4,4'-difluorodiphenyl sulfone: same as Example 1
[0046] (3) Synthesis of 3,3'-diethynyl-4,4'-difluorodiphenylsulfone: Same as Example 1
[0047] (4) Add 0.5g of 3,3'-diethynyl-4,4'-difluorodiphenyl sulfone into a round bottom flask, fill with nitrogen, and add 0.0264g of CuSO in turn under the protection of nitrogen 4 , 1.64gNaAsc, 5mlTHF and 0.476g azidotrimethylsilane, point the plate to observe the reaction process until the reaction is complete, add ethyl acetate and water layer, the organic layer is washed with 5% ammonia water, anhydrous MgSO 4 Dried, filtered and spin-dried, and separated by column chromatography to obtain triazole-functionalized 4,4'-difluorodiphenylsulfone.
[0048] The compound synthesized in the present invention can be polycondensed with bisphenol compounds to obtain polymer polysulfone material. The polys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com