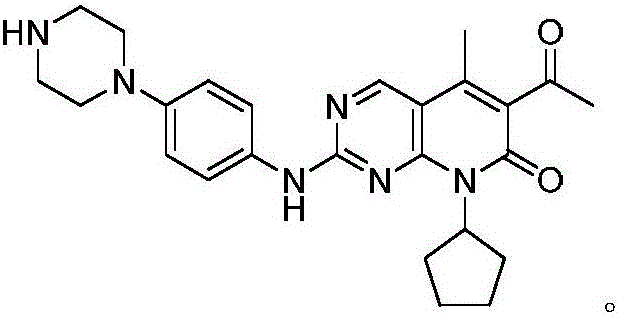
Method for preparing CDK46 kinase inhibitor Palbociclib
A technology of kinase inhibition and cyclopentyl group, applied in the field of drug synthesis, can solve the problems of long reaction time, low overall yield, unfavorable health of workers, etc., and achieve the effects of mild reaction conditions and high overall yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A method for preparing CDK46 kinase inhibitor palbociclib, the method comprising the following steps:
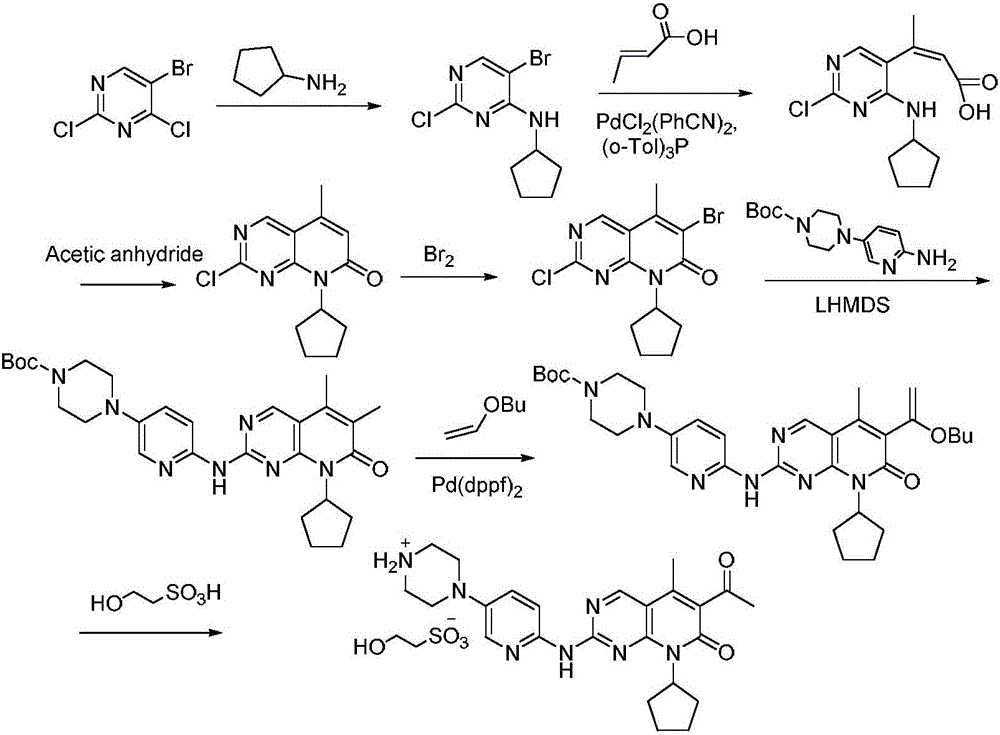
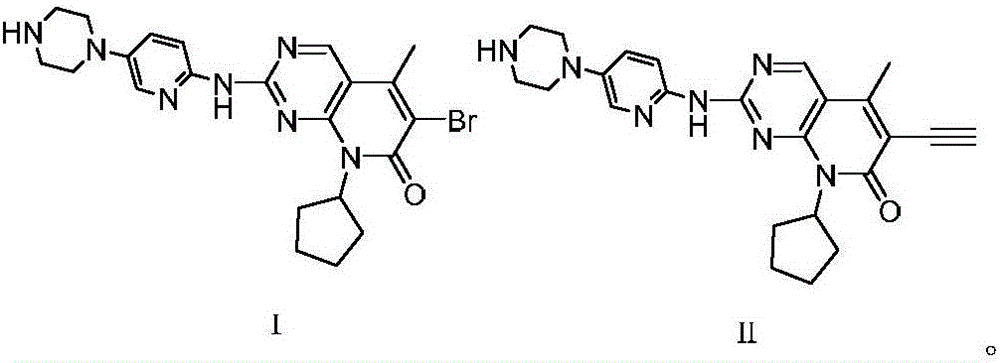
[0031] 1) Under nitrogen protection, the compound N-cyclopentyl-5-methyl-2-[[5-(1-piperazinyl)-2-pyridyl]amino]-6- Bromopyrido[2,3-d]pyrimidin-7(8H)-one 48.4g (100mmol) in the presence of cuprous bromide 5.7g (40mmol) and potassium tert-butoxide 28g (250mmol) with trimethylsilyl Acetylene 16.7g (170mmol) was reacted in THF at 55°C for 3 hours. After the reaction was completed, the solvent was evaporated under reduced pressure, washed with water, recrystallized from methanol, and dried to obtain the compound N-cyclopentyl-5-methanone represented by formula (II) Base-6-ethynyl-2-[[5-(1-piperazinyl)-2-pyridyl]amino]pyrido[2,3-d]pyrimidin-7(8H)-one 34.4g, yield It is 80.2%, and the purity is 99.90% (HPLC area normalization method).
[0032] 2) The compound N-cyclopentyl-5-methyl-6-ethynyl-2-[[5-(1-piperazinyl)-2-pyridyl) represented by the formula (II) obtained in step ...
Embodiment 2
[0035] A method for preparing CDK46 kinase inhibitor palbociclib, the method comprising the following steps:
[0036] 1) Under nitrogen protection, the compound N-cyclopentyl-5-methyl-2-[[5-(1-piperazinyl)-2-pyridyl]amino]-6- Bromopyrido[2,3-d]pyrimidin-7(8H)-one 48.4g (100mmol) in the presence of cuprous bromide 5.7g (40mmol) and potassium tert-butoxide 28g (200mmol) with trimethylsilyl Acetylene 17.7g (180mmol) was reacted in THF at 65°C for 4 hours. After the reaction was completed, the solvent was evaporated under reduced pressure, washed with water, recrystallized from methanol, and dried to obtain the compound N-cyclopentyl-5-methanone represented by formula (II) Base-6-ethynyl-2-[[5-(1-piperazinyl)-2-pyridyl]amino]pyrido[2,3-d]pyrimidin-7(8H)-one 34.2g, yield It is 79.7%, and the purity is 99.90% (HPLC area normalization method).
[0037] 2) The compound N-cyclopentyl-5-methyl-6-ethynyl-2-[[5-(1-piperazinyl)-2-pyridyl) represented by the formula (II) obtained in step ...
Embodiment 3
[0039] A method for preparing CDK46 kinase inhibitor palbociclib, the method comprising the following steps:
[0040]1) Under nitrogen protection, the compound N-cyclopentyl-5-methyl-2-[[5-(1-piperazinyl)-2-pyridyl]amino]-6- Bromopyrido[2,3-d]pyrimidin-7(8H)-one 48.4g (100mmol) in the presence of cuprous bromide 7.2g (50mmol) and potassium tert-butoxide 33.7g (300mmol) with trimethylsilane 14.7g (150mmol) of acetylene was reacted in THF at 60°C for 3 hours. After the reaction, the solvent was evaporated under reduced pressure, washed with water, recrystallized from methanol, and dried to obtain the compound N-cyclopentyl-5- Methyl-6-ethynyl-2-[[5-(1-piperazinyl)-2-pyridyl]amino]pyrido[2,3-d]pyrimidin-7(8H)-one 33.9g, received The yield was 78.9%, and the purity was 99.90% (HPLC area normalization method).
[0041] 2) The compound N-cyclopentyl-5-methyl-6-ethynyl-2-[[5-(1-piperazinyl)-2-pyridyl) represented by the formula (II) obtained in step 1) ]Amino]pyrido[2,3-d]pyrimidi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com