Continuous synthesis system and method for trimethylsilylacetylene
A trimethylsilyl-based, chemical synthesis technology, applied in the direction of silicon organic compounds, chemical instruments and methods, compounds of group 4/14 elements of the periodic table, etc., can solve the potential risk of increasing operation and the risk of reaction amplification, Grignard Long reagent production cycle, increased acetylene gas loss and other issues, to achieve the effect of lower safety hazards, shorten production cycle, and save production time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
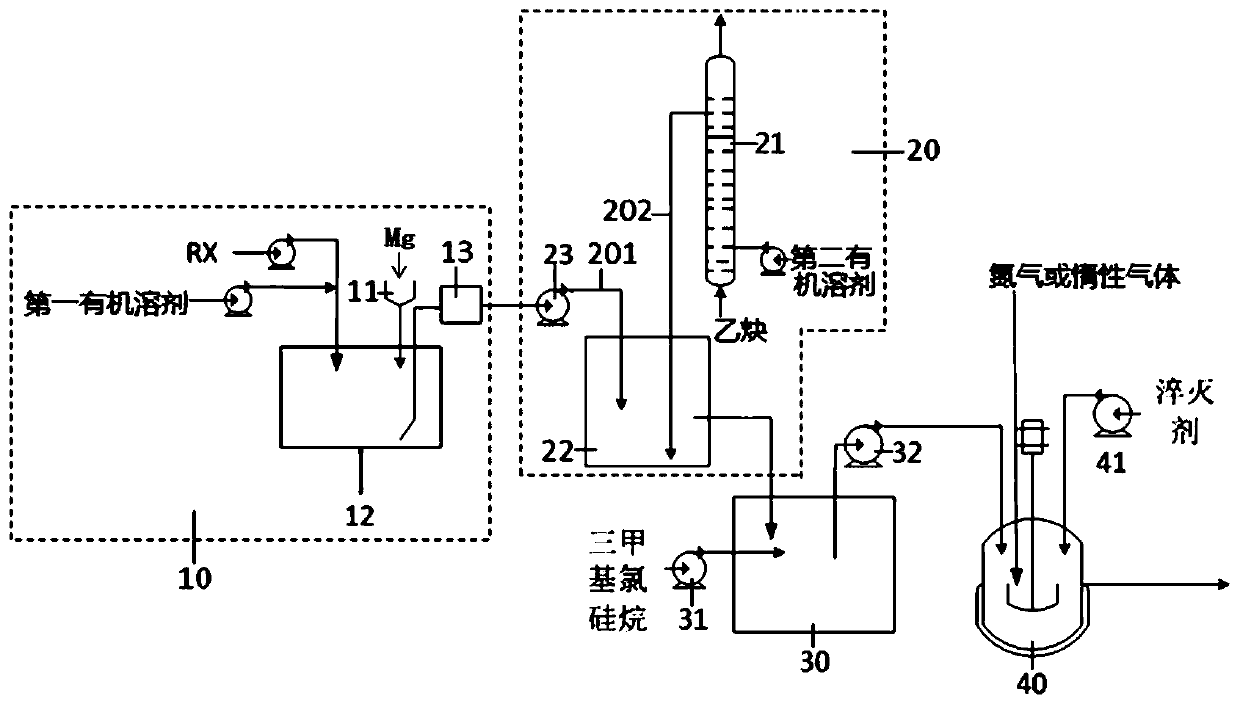
[0064]Another aspect of the present application also provides a continuous synthesis method of trimethylsilylacetylene, the continuous synthesis method adopts the above continuous synthesis system to prepare trimethylsilylacetylene, the continuous synthesis method comprises: making halogen Substituted hydrocarbon, the first organic solvent and metal magnesium are continuously delivered to the Grignard reagent continuous preparation unit 10 for continuous Grignard reagent preparation reaction to obtain the Grignard reagent; the Grignard reagent and acetylene solution are continuously delivered to the acetylene Grignard reagent continuous The synthesis unit 20 carries out the continuous synthesis reaction of the acetylene Grignard reagent to obtain the acetylene Grignard reagent; The continuous preparation reaction of acetylene gives trimethylsilyl acetylene.
[0065] The continuous synthesis system of trimethylsilylacetylene can realize the continuous preparation of Grignard re...
Embodiment 1
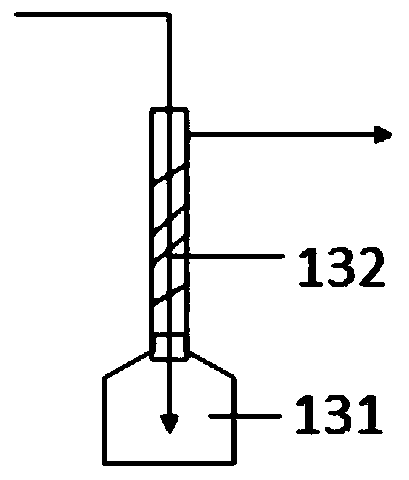
[0084] 5 g of chlorobutane, 15 g of the first organic solvent (tetrahydrofuran), and 1.5 g of metallic magnesium were added to the 500 ml Grignard reagent reaction device 12 in advance. The temperature in the Grignard reagent reaction device 12 is controlled by a first temperature control device, wherein an oil bath is arranged in the first temperature control device. Heat the oil bath to 50°C and keep it warm for 10 minutes. A drop of initiator (1,2-dibromoethane) is added dropwise into the Grignard reagent reaction device 12 to initiate the Grignard reagent preparation reaction. After observing that the temperature in the Grignard reagent reaction unit 12 has obvious promotion, open the first feed pump 14, add chlorobutane with the speed of 1g / min; Open the second feed pump 15, with the speed of 3g / min Add tetrahydrofuran; open the solid feeder, and add magnesium at a rate of 0.29 g / min. At the same time, the first temperature control device is used to keep the temperature...
Embodiment 2
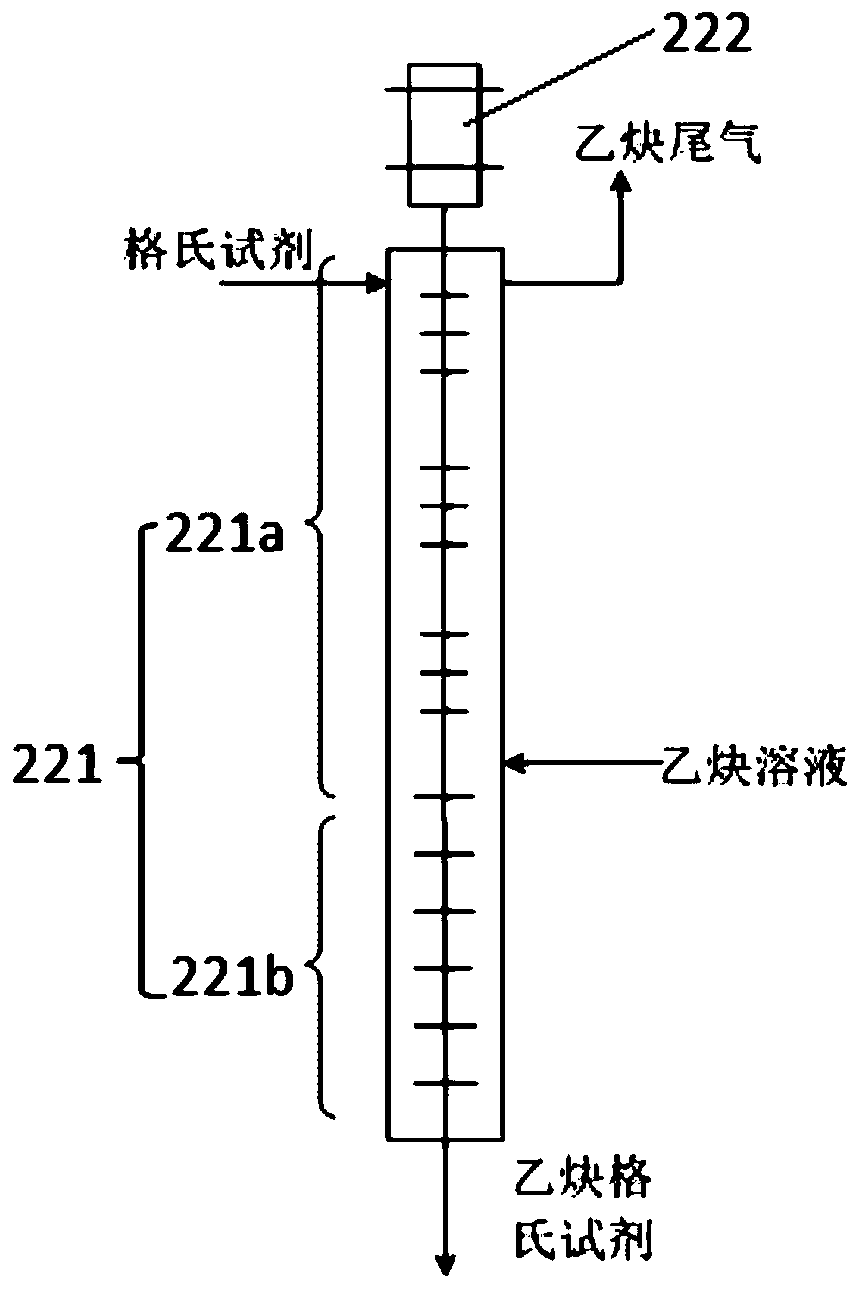
[0088]The difference from Example 1 is that the acetylene solution overflow pipe is suspended, and is not inserted into the bottom of the acetylene Grignard reagent synthesis device 22 .
[0089] 5 g of chlorobutane, 15 g of the first organic solvent (tetrahydrofuran), and 1.5 g of metallic magnesium were added to the 500 ml Grignard reagent reaction device 12 in advance. The temperature in the Grignard reagent reaction device 12 is controlled by a first temperature control device, wherein an oil bath is arranged in the first temperature control device. Heat the oil bath to 50°C and keep it warm for 10 minutes. A drop of initiator (1,2-dibromoethane) is added dropwise into the Grignard reagent reaction device 12 to initiate the Grignard reagent preparation reaction. After observing that the temperature in the Grignard reagent reaction unit 12 has obvious promotion, open the first feed pump 14, add chlorobutane with the speed of 1g / min; Open the second feed pump 15, with the s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com