Synthetic method of 7-azaindole-5-chloro-6-carboxylic acid
A synthesis method and azaindole technology are applied in the field of azaindole heterocyclic compounds, can solve problems such as no industrialized effective synthesis method, etc., and achieve the effects of simple reaction conditions, cheap reagents and easy purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
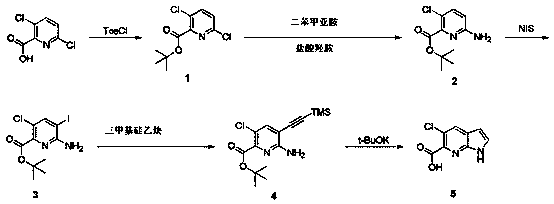
[0016] Example 1: Preparation of 7-azaindole-5-chloro-6-carboxylic acid.
[0017] step 1:
[0018] 3,6-Dichloropyridine-2-carboxylic acid (78.3 g, 0.408 mol) was dissolved in tert-butanol (780 mL) and pyridine (215 mL), and cooled to 0°C in an ice-water bath. P-toluenesulfonyl chloride (185 g, 0.97 mol) was added in portions, and after the addition was completed, the mixture was raised to room temperature and stirred overnight. The reaction solution was concentrated to dryness, and the residue was added to 5% aqueous citric acid solution (2 L) and extracted with ethyl acetate (2 L). The organic phase was washed with 5% sodium bicarbonate aqueous solution (500 mL) and saturated brine (500 mL), and the organic phase was dried over anhydrous sodium sulfate, filtered, and concentrated to dryness to obtain compound 1 (100 g, 98% yield). 1 H NMR (400 MHz, CDCl 3 )1.63 (s, 9H), 7.35 (d, J = 8.4 Hz, 1H), 7.71 (d, J = 8.4 Hz, 1H).
[0019] Step 2:
[0020] Compound 1 (50 g, 0...
Embodiment 2
[0027] Example 2, the coupling reaction temperature in step 2 is 85°C, and the reaction time is 2 hours; the reaction temperature in step 3 is 50°C; the reaction temperature in step 4 is 20°C, and the reaction time is 2 hours; the reaction temperature in step 5 is 60°C. All the other are with embodiment 1.
Embodiment 3
[0028] Example 3, the coupling reaction temperature in step 2 is 95°C, and the reaction time is 1 hour; the reaction temperature in step 3 is 60°C; the reaction temperature in step 4 is 35°C, and the reaction time is 0.5 hour; the reaction temperature in step 5 is 70°C. All the other are with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com