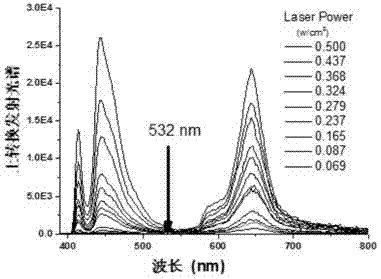
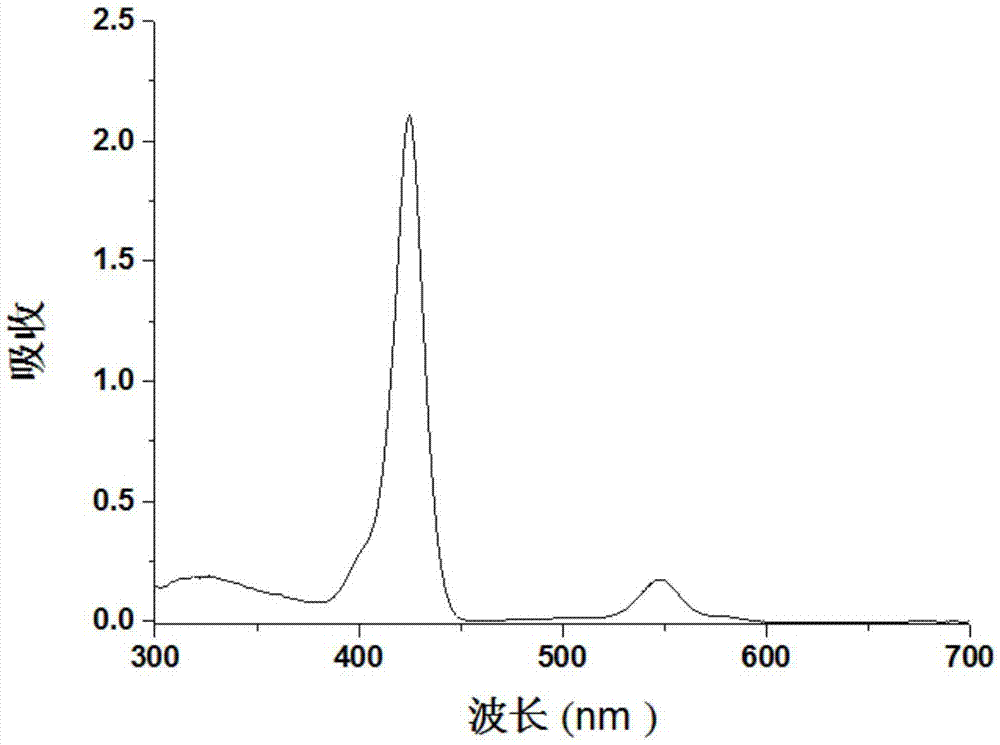
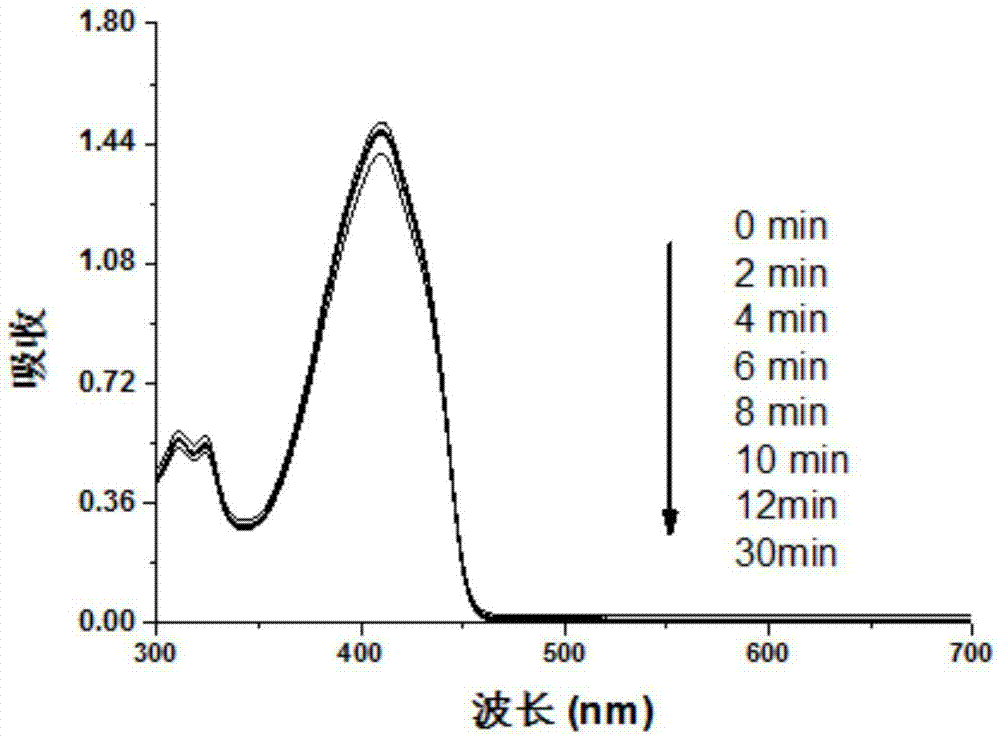
Preparation method for metalloporphyrin metal complexes with different structure frameworks and application
A technology of metal complexes and complexes, applied in chemical instruments and methods, color/spectral characteristic measurement, luminescent materials, etc., can solve problems such as high excitation light energy, difficult adjustment of photosensitizer performance, and low up-conversion quantum efficiency. Achieve long triplet lifetime, strong visible light and near-infrared light absorption ability, rich photophysical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Complex P 1 Synthesis
[0031]
[0032] (1) Synthesis of 4-trimethylsilylethynyl benzaldehyde
[0033] Add p-bromobenzaldehyde (1.85g, 10mmol), Pd(PPh 3 ) 4 (0.35g, 0.3mmol), CuI (57mg, 0.3mmol). Install the reflux device to seal and vacuum drum N 2 , Add drum N 2 15min of diisopropylamine (5ml) and trimethylsilylacetylene (2.0ml, 15mmol). The condensed water was refluxed and reacted with stirring at 80°C for 2 hours. After the reaction, silica gel powder was added and spin-dried to form a powder. The sample was applied to the column by the dry method of dichloromethane: petroleum ether (5:1) and spin-dried to obtain a white solid. Yield: 85%. 1 HNMR(400MHz, CDCl 3 )δ10.00(s,1H), 7.83–7.80(m,2H), 7.62–7.59(m,2H), 0.28–0.26(m,9H).
[0034] (2)Z 1 Compound preparation
[0035] Add pentafluorobenzaldehyde (1.48g, 7.5mmol) and 4-trimethylsilylethynylbenzaldehyde (0.51g, 2.5mmol) into a 1000ml two-necked flask. After sealing, vacuum drum nitrogen is applied and repeate...
Embodiment 2
[0042] Example 2: Complex P 2 Synthesis
[0043]
[0044] (1) Compound Z 4 Synthesis
[0045] Add Z to the two-neck bottle 2 (91mg, 0.1mmol), Pd(PPh 3 ) 4 (3.5mg, 0.003mmol), 9,10-dibromoanthracene (8.4mg, 0.025mmol) is sealed and vacuumed and bubbling with nitrogen three times, bubbling for 15min with 8ml of toluene and 2ml of triethylamine. The condensed water was refluxed and reacted at 80°C for 48 hours. After the reaction, silica gel powder was added and spin-dried to form a powder. The sample was applied to the column by the dry method of dichloromethane: petroleum ether (8:1) and spin-dried to obtain an orange solid. 1 HNMR(400MHz, CDCl 3 )δ9.09(d,J=4.6Hz,4H), 8.98(dd,J=6.7,3.3Hz,4H), 8.91(dd,J=11.1,4.0Hz,12H), 8.36(d,J=8.1 Hz, 4H), 8.27 (d, J = 8.1 Hz, 4H), 7.84 (dd, J = 6.8, 3.1 Hz, 4H), -2.80 (s, 4H).
[0046] (2) Complex P 2 Synthesis
[0047] Add Z to the two-neck bottle 4 (50mg, 0.025mmol) zinc acetate (183mg, 1mmol) was added to 3ml of dichloromethane and 6ml of methano...
Embodiment 3
[0048] Example 3: Compound P 3 Synthesis
[0049]
[0050] (1) Compound Z 7 Synthesis
[0051] Add pentafluorobenzaldehyde (1.48g, 7.5mmol) and p-iodobenzaldehyde (0.58g, 2.5mmol) into a 1000ml two-necked flask, seal and evacuate nitrogen and repeat three times. Add 800ml of redistilled dichloromethane, then inject pyrrole (1.04ml, 15mmol), bubbling with nitrogen for 15min, add boron trifluoride ether (1.2ml, 5mmol) and the solution will slowly turn red. After one hour, add two Chlorodicyanobenzoquinone (DDQ) (1.20g, 5mmol) will turn black. After two hours, 0.8 ml of triethylamine was added. Two hours later, it was filtered and the filtrate was collected and spin-dried through the column. Dichloromethane: petroleum ether (15:1) was applied to the column by dry method and spin-dried to obtain a purple-red solid. 1 HNMR(400MHz, CDCl 3 )δ8.86(ddd,J=18.5,14.2,4.8Hz,8H), 8.12(dd,J=8.4,2.0Hz,4H),7.97–7.92(m,4H),-2.86(d,J=22.7 Hz, 2H).
[0052] (2) Compound Z 5 Synthesis
[0053] Add Z t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com