Synthesis method of 3-hydroxy-4-((trimethylsilyl) ethynyl) methyl benzoate
A technology of trimethylsilyl and methyl benzoate, which is applied in the field of synthesis of pharmaceutical intermediates, can solve problems such as product quality, unstable yield, products that cannot meet market demand, and difficulty in adapting to market competitiveness. The quality of recovered solvent is stable, the rate of catalytic synthesis is improved, and the effect of reducing the amount of catalyst
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
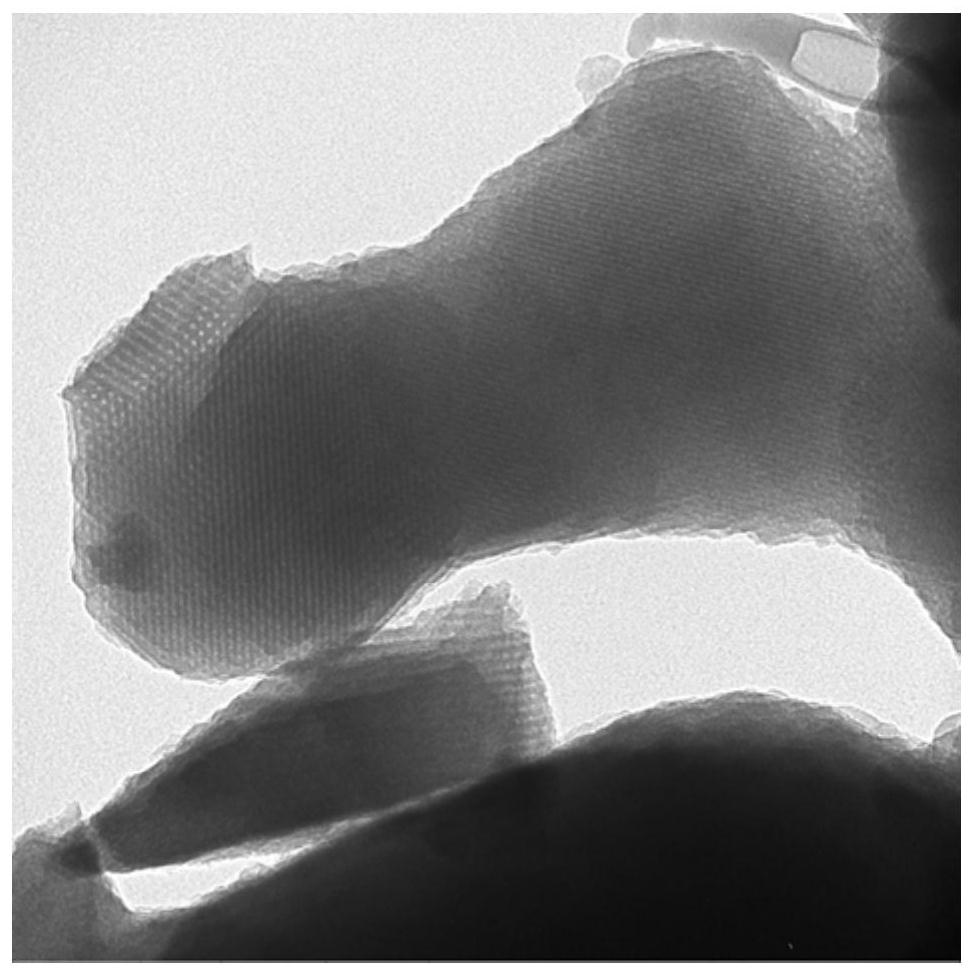
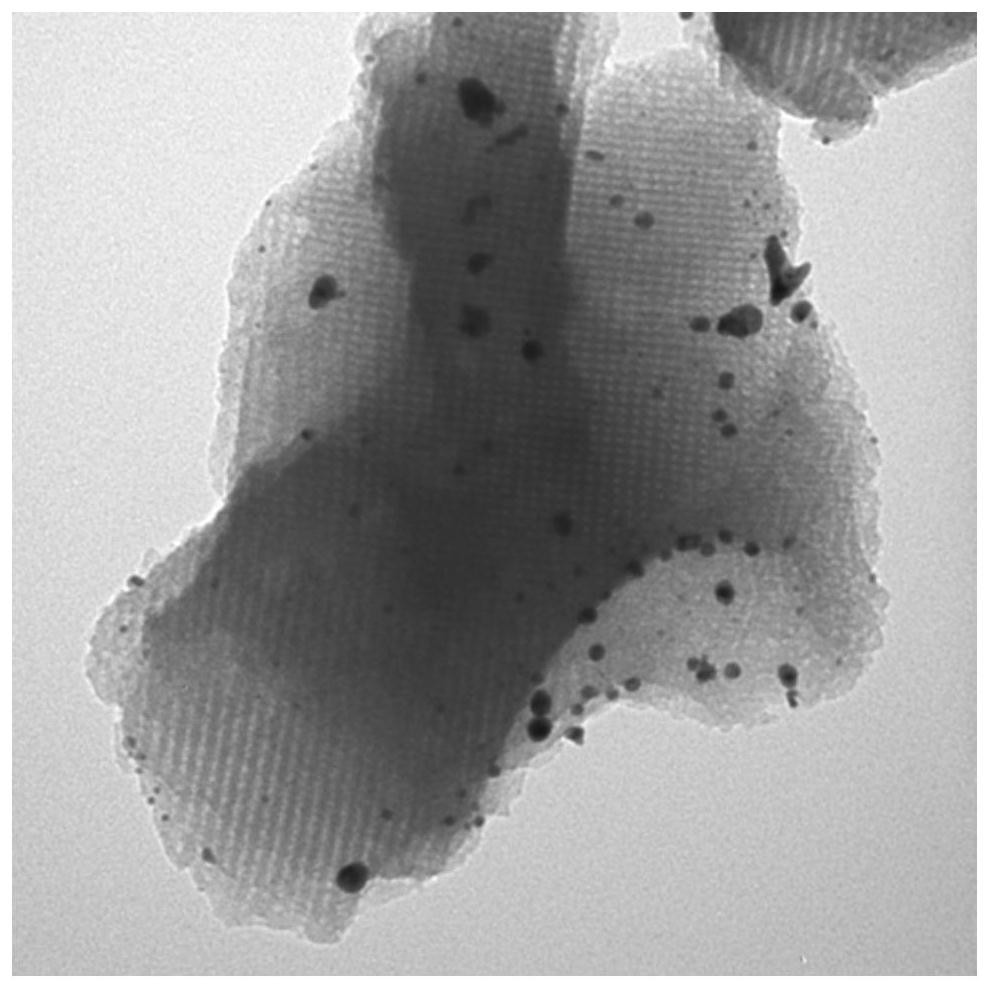
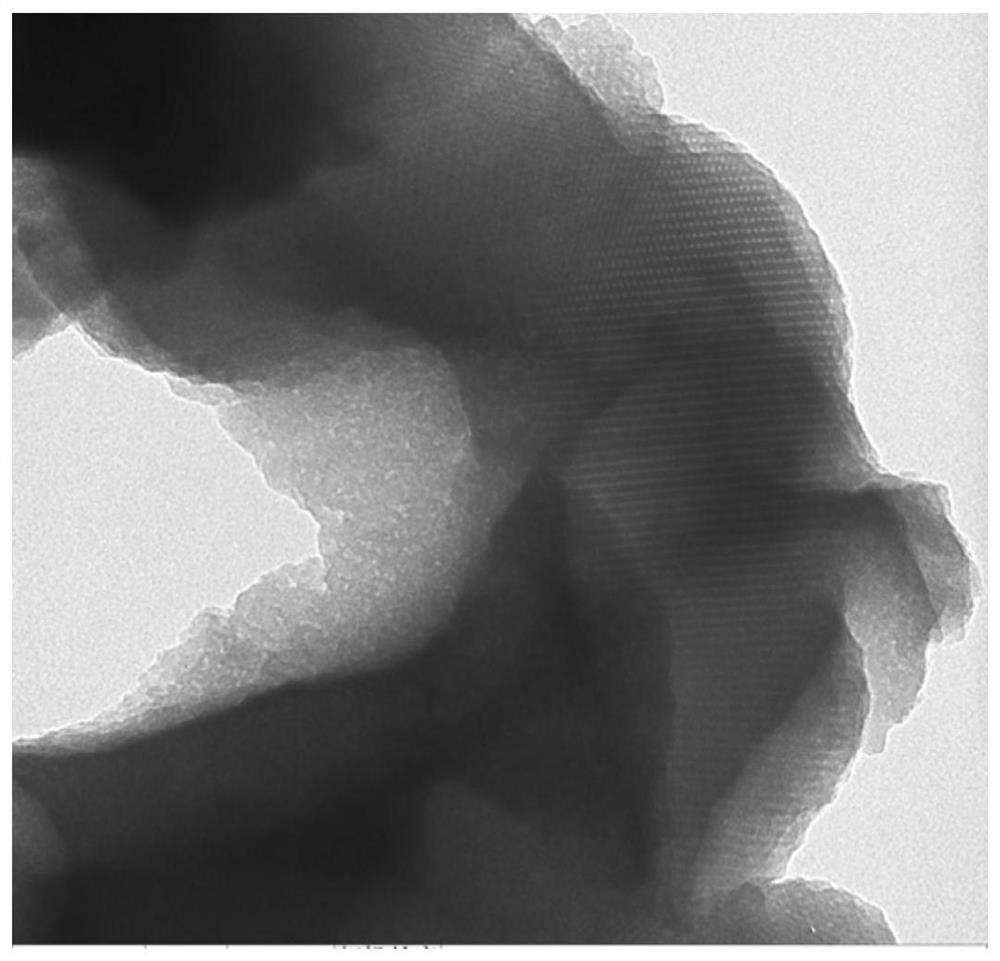
Image
Examples
Embodiment 1
[0042] 1) Add 5g of phenol into a 50ml single-necked round bottom flask to melt it; add 0.75ml of ammonia dropwise and then add 8.86g of formaldehyde solution with a mass fraction of 37% dropwise; react at 80°C for 75 minutes; and use 0.6mol / L hydrochloric acid solution to adjust the pH value of the solution to neutral, and vacuum rotary evaporation dehydration to obtain 3.67 g of A-stage phenolic resin.
[0043] The obtained product A-stage phenolic resin is diluted with ethanol; 1g of F127 is placed in a 50ml round bottom flask and 20ml of ethanol is added to dissolve the F127, and the A-stage phenolic resin diluted with ethanol is added dropwise; the ethanol in the mixture is completely volatilized; Thermally polymerized at 100°C, and finally calcined the polymer at 350°C for 6 hours to obtain a catalyst carrier.
[0044] 2 g of the catalyst carrier was added to deionized water, and 0.2 g of palladium dichloride was added, and stirred at room temperature for 3 h to obtain a...
Embodiment 2
[0049] 1) Add 5g of phenol into a 50ml single-necked round bottom flask to melt it; add 0.75ml of ammonia dropwise and then add 8.86g of formaldehyde solution with a mass fraction of 37% dropwise; react at 80°C for 75 minutes; and use 0.6mol / L hydrochloric acid solution to adjust the pH value of the solution to neutral, and vacuum rotary evaporation dehydration to obtain 3.71 g of A-stage phenolic resin.
[0050] The obtained product A-stage phenolic resin is diluted with ethanol; 1g P123 is placed in a 50ml round-bottomed flask and 20ml of ethanol is added to dissolve P123, and the A-stage phenolic resin diluted with ethanol is added dropwise; ethanol is completely volatilized in the mixture; Thermally polymerized at 100°C, and finally calcined the polymer at 550°C for 4.5 hours to obtain a catalyst carrier.
[0051] 2 g of the catalyst carrier was added to ethanol, and 0.2 g of palladium dichloride was added, and stirred at room temperature for 3 h to obtain a palladium-supp...
Embodiment 3
[0056] 1) Add 5g of phenol into a 50ml single-necked round bottom flask to melt it; add 0.75ml of ammonia water dropwise and then add 8.86g of formaldehyde solution with a mass fraction of 37% dropwise; react at 80°C for 75 minutes; and use 0.6mol / L hydrochloric acid solution to adjust the pH value of the solution to neutral, and vacuum rotary evaporation and dehydration to obtain 3.63 g of A-stage phenolic resin.
[0057] The obtained product A-stage phenolic resin is diluted with ethanol; 1g F108 is placed in a 50ml round-bottomed flask and 20ml of ethanol is added to dissolve the F108, and the A-stage phenolic resin diluted with ethanol is added dropwise; ethanol is completely volatilized in the mixture; Thermally polymerized at 100°C, and finally calcined the polymer at 650°C for 3 hours to obtain a catalyst carrier.
[0058] Add 2 g of the catalyst carrier into methanol, and add 0.2 g of palladium dichloride, and stir at room temperature for 3 h to obtain a palladium-supp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com