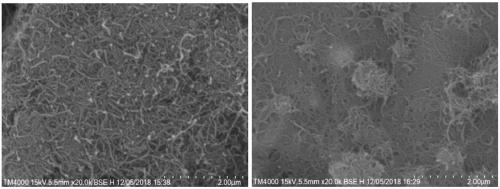
Carbon nanotube dispersing phenylacetylene-based polyfunctional group polymer and preparation method thereof
A technology of functional polymers and carbon nanotubes, applied in the field of phenylacetylene-based polyfunctional polymers and their preparation, can solve the problems of restricting the use of carbon nanotubes, agglomeration of carbon nanotubes, entanglement or knotting, etc., and it is convenient to achieve process parameters. The effect of simple control and synthesis method and low cost of use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
[0032] figure 1 Preparation method of phenylethynyl polyfunctional polymer 5a.
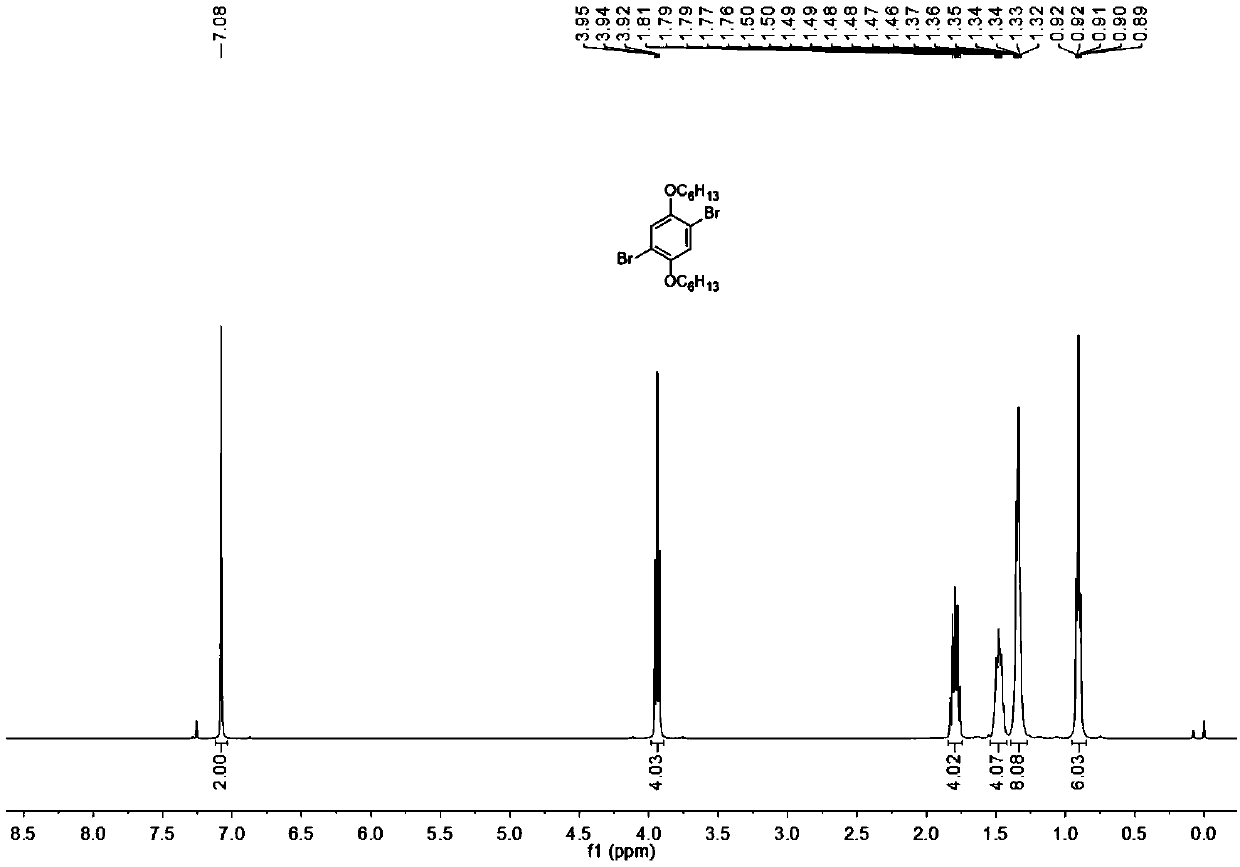
[0033] Intermediate 2a: Add 1,3-dibutoxybenzene (22.2g, 100mmol) to a dry 1000Ml three-neck flask placed in an ice-salt bath at -5°C, add 500Ml of dry dichloromethane, and stir for 20 minutes Then add bromine water (40.0g, 250mmol) dichloromethane solution 200Ml dropwise, dropwise in 60 minutes, remove the ice-salt bath after dropping, stir at room temperature for 24 hours, add an appropriate amount of sodium bicarbonate solution to neutralize the reaction system, Then it was extracted, dried, concentrated by rotary evaporation, and purified by column chromatography to obtain 31.4 g of white powdery solid with a yield of 83%. 1 H NMR (400MHz, Chloroform-d) δ7.08(s, 2H), 3.94(t, J=6.5Hz, 4H), 1.85–1.70 (m, 4H), 1.58–1.45(m, 4H), 0.98( t,J=7.4Hz,6H). 13 C NMR (100MHz, Chloroform-d) δ150.1, 118.5, 111.2, 70.0, 31.2, 19.2, 13.8.
[0034] Intermediate 3a: Under nitrogen protection, add ...
Embodiment 2
[0038]
[0039] Intermediate 2b: Add 1,3-dihexyloxybenzene (27.8 g, 100 mmol) into a dry 1000Ml three-neck flask placed in an ice-salt bath at -5°C, add 500Ml of dry dichloromethane, and stir for 20 minutes Add 200Ml of dichloromethane solution of bromine water (40.0g, 250mmol) dropwise, drop it in 60 minutes, remove the ice-salt bath after dropping, stir at room temperature for 24 hours, add an appropriate amount of sodium bicarbonate solution to neutralize the reaction system, Then it was extracted, dried, concentrated by rotary evaporation, and purified by column chromatography to obtain 36.9 g of white powdery solid with a yield of 85%. 1 H NMR (400MHz, Chloroform-d) δ7.08(s,2H),3.94(t,J=6.5Hz,4H), 1.86–1.71(m,4H),1.61–1.41(m,4H),1.40– 1.24(m,8H),0.97–0.79(m,6H). 13 CNMR (100 MHz, Chloroform-d) δ150.1, 118.4, 111.1, 70.3, 31.5, 29.1, 25.6, 22.6, 14.0.
[0040] Intermediate 3b: Under nitrogen protection, add Intermediate 2b (8.7g, 20mmol) to a dry 250Ml three-necked fl...
Embodiment 3
[0044]
[0045] Intermediate 2c: Add 1,3-dioctyloxybenzene (33.4 g, 100 mmol) into a dry 1000Ml three-necked flask placed in an ice-salt bath at -5°C, add 500Ml of dry dichloromethane, and stir for 20 minutes Add 200Ml of dichloromethane solution of bromine water (40.0g, 250mmol) dropwise, drop it in 60 minutes, remove the ice-salt bath after dropping, stir at room temperature for 24 hours, add an appropriate amount of sodium bicarbonate solution to neutralize the reaction system, Then it was extracted, dried, concentrated by rotary evaporation, and purified by column chromatography to obtain 38.7 g of white powdery solid with a yield of 79%. 1 H NMR (400MHz, Chloroform-d) δ7.07(s,2H),3.92(t,J=6.5Hz,4H), 1.85–1.73(m,4H),1.59–1.39(m,4H),1.41– 1.21(m,16H),0.95–0.77(m,6H). 13 C NMR (100 MHz, Chloroform-d) δ150.1, 118.4, 111.0, 71.4, 31.7, 29.5, 28.5, 27.1, 25.7, 22.6, 14.0.
[0046] Intermediate 3c: Under nitrogen protection, add Intermediate 2c (9.8g, 20mmol) to a dry 250Ml...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com