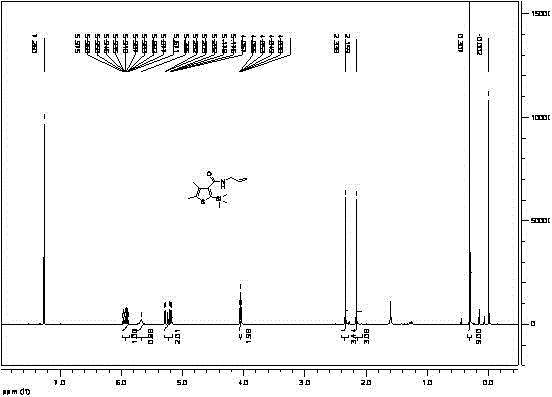
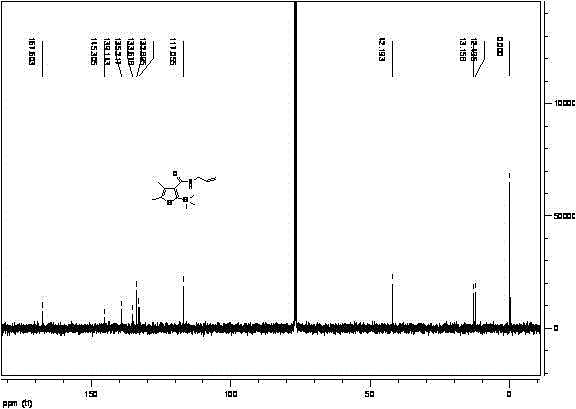
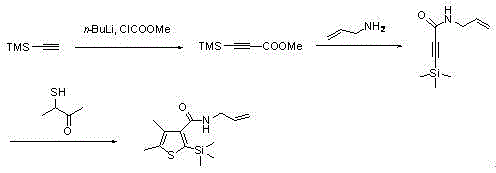Synthetic method for silthiopham
A technology of silthiofacillin and synthetic method, which is applied in the field of organic synthesis, can solve the problems of many by-products, cumbersome synthesis process, environmental pollution, etc., and achieve the effect of simple route, cheap and easy-to-obtain raw materials, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) The method for preparing methyl trimethylsilyl propiolate is:
[0028] Under nitrogen protection, trimethylsilylacetylene (4.0mL, 28.6mmol) was added to dry THF (20mL), cooled to -78°C, and n-butyllithium (18.0mL, 28.6mmol) was slowly added to the system , and stirred at this temperature for 30 minutes, then warmed to room temperature, and added methyl chloroformate (2.4 mL, 31.4 mmol), followed by stirring the reaction for 2.5 hours. The reaction mixture was poured into ammonium chloride solution to quench the reaction, and extracted with diethyl ether, the organic phase was washed with water and brine and dried with anhydrous sodium sulfate, the solvent (tetrahydrofuran and diethyl ether) was removed in vacuo, and the obtained crude product was purified by column chromatography ( 5%EtOAc / PE), to obtain yellow oil product methyl trimethylsilylpropiolate 4.2g, yield 95.0%;
[0029] (2) Preparation N -Allyl-3-(trimethylsilyl)propynamide
[0030] Under nitrogen pro...
Embodiment 2
[0036] (1) Preparation of ethyl trimethylsilyl propiolate
[0037] Under nitrogen protection, trimethylsilylacetylene (5.6mL, 40mmol) was added to dry THF, cooled to -65°C, tert-butyllithium (32.62mL, 52.0mmol) was slowly added to the system, and at this temperature It was stirred for 30 minutes, then warmed to room temperature, and ethyl chloroformate (6.48 g, 60 mmol) was added, followed by stirring for 2.5 hours. The reaction mixture was poured into ammonium chloride solution to quench the reaction, and extracted with diethyl ether. The organic phase was washed with water and brine and dried over anhydrous sodium sulfate. The solvent was removed in vacuo, and the obtained crude product was purified by column chromatography (5% EtOAc / PE) to obtain 6.34 g of methyl trimethylsilylpropiolate as a yellow oil product, with a yield of 93.0%.
[0038] (2) Preparation N -Allyl-3-(trimethylsilyl)propynamide
[0039] Under nitrogen protection, 30% mol catalyst tetraisopropyl titana...
Embodiment 3
[0044] (1) Preparation of methyl trimethylsilyl propiolate
[0045] Under nitrogen protection, trimethylsilylacetylene (4.0mL, 28.6mmol) was added to dry THF (20mL), cooled to -50°C, methyllithium (26.86mL, 60mmol) was slowly added to the system, and in Stir at this temperature for 30 minutes, then warm to room temperature, and add methyl chloroformate (2.03 mL, 37.2 mmol), then stir the reaction for 2.5 hours. The reaction mixture was poured into ammonium chloride solution to quench the reaction, and extracted with diethyl ether. The organic phase was washed with water and brine and dried over anhydrous sodium sulfate. The solvent was removed in vacuo, and the obtained crude product was purified by column chromatography (5% EtOAc / PE) to obtain 4.1 g of methyl trimethylsilylpropiolate as a yellow oil product, with a yield of 93.0%.
[0046] (2) Preparation N -Allyl-3-(trimethylsilyl)propynamide
[0047] Under the protection of nitrogen, the catalyst zirconium tetra-tert-but...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com