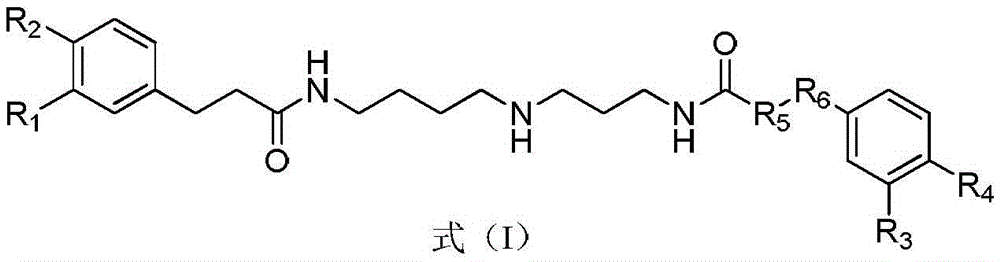
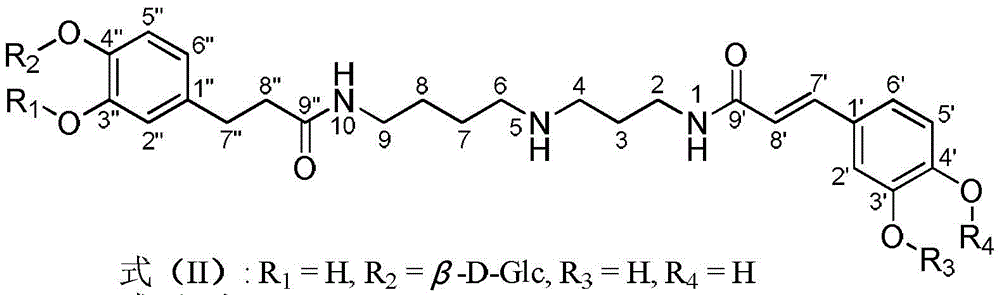
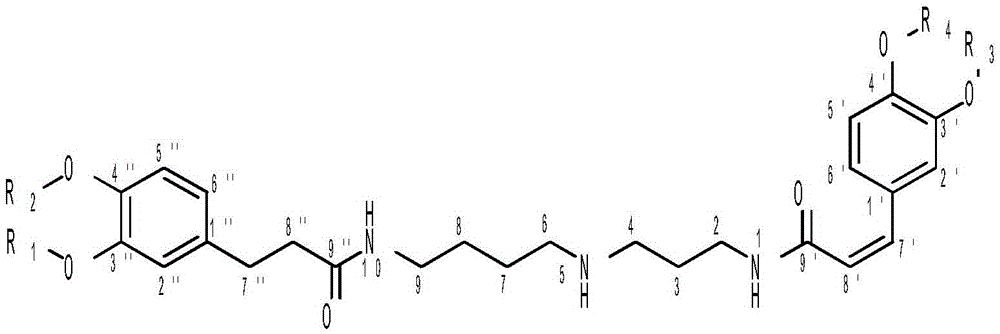
Dicaffeoyl-spermidine cyclization derivative glycoside and application thereof
一种咖啡酰亚精胺、衍生物的技术,应用在糖衍生物、糖衍生物、糖衍生物制备等方向,能够解决研究少、二咖啡酰亚精胺衍生物稀少植物成分等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Embodiment 1 formula (II) - the preparation of formula (XIV) compound
[0070] 19.5kg wolfberry was heated and refluxed with 100L ethanol-water (60:40, v / v) to extract 3 times, 2 hours each time. After filtration, the filtrate was concentrated under reduced pressure to obtain a concentrate. The concentrated solution was chromatographed on a macroporous resin column, and eluted with ethanol-water with a volume ratio of 0:100, 30:70, 50:50, 70:30 and 95:5 in sequence to obtain 5 fractions F1, F2, and F3 , F4, F5. Next, for the fraction F2 obtained by the ethanol-water elution with a volume ratio of 30:70, 70.0 grams of F2 were taken for normal pressure silica gel column chromatography, and the volume ratio was 95:5:0, 90:10:1, 85 :15:1.5, 80:20:2, 70:30:3, 60:40:4, 50:50:5, 40:60:6 and 0:100:0 in chloroform-methanol-water elution to give F2.1, F2.2, F2.3, F2.4, F2.5, F2.6, F2.7, F2.8, F2.9 and F2.10 are 10 sub-fractions. Then the sub-fraction F2.8 (10.1g) obtained by ...
Embodiment 2
[0105] Example 2, results of glycoside antioxidant activity of dicaffeoylspermidine derivatives
[0106] The antioxidant activity of the compound is evaluated by the oxygenradicalabsorbancecapacity (ORAC) test, and the specific experimental procedure is as follows. Add 0.248g AAPH (2,2'-azobisisobutylamidine dihydrochloride) into 50mL phosphate buffer system to prepare 18.3mM AAPH stock solution. Add 20mL of phosphate buffer solution, 20mL of the sample to be tested or standard substance Trolox solution (concentration: 6.25mM) and 20mL fluorescent substance disodiumfluorescein (FL, concentration: 630nM) into the wells of the 96-well plate in sequence. Next, quickly add 140 mLAAPH (18.3 mM concentration) to the wells of the 96-well plate, and immediately place the 96-well plate in the GENios Luciferase-based microplate reader produced by Tecan, Switzerland, and set the excitation wavelength to 485 nm. , the emission wavelength is 527nm, and the fluorescence intensity is measur...
Embodiment 3
[0114] Embodiment 3, compounds improve the learning and memory activity test method of senile dementia Drosophila
[0115] (1) Cultivation of Alzheimer's Drosophila
[0116] w 1118 (isoCJ1) was used as the control group of the experiment, the background Drosophila, abbreviated as "2U". Successful transfer into pathogenic Aβ 42 The Drosophila protein is (UAS-Aβ 42 ; abbreviated as "H29.3"). This strain of Drosophila was crossed with Drosophila expressing the Gal4 promoter in the whole brain to obtain elav-GAL4 c155 (P35) and Aβ 42 Drosophila strains.
[0117] (2) Administration of Alzheimer's Drosophila
[0118] Three groups were set up in the experiment: healthy fruit flies without drug control, diseased fruit flies without drug control and diseased fruit flies with administration.
[0119] The parents of all tested fruit flies were reared and bred in a fly house with a constant temperature of 24° C. and a constant humidity of 42% RH (Relativehumidity). On the first d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com