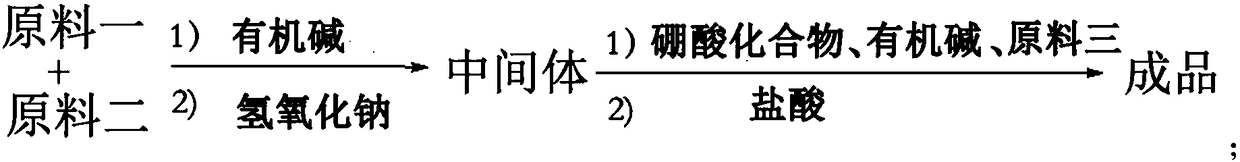
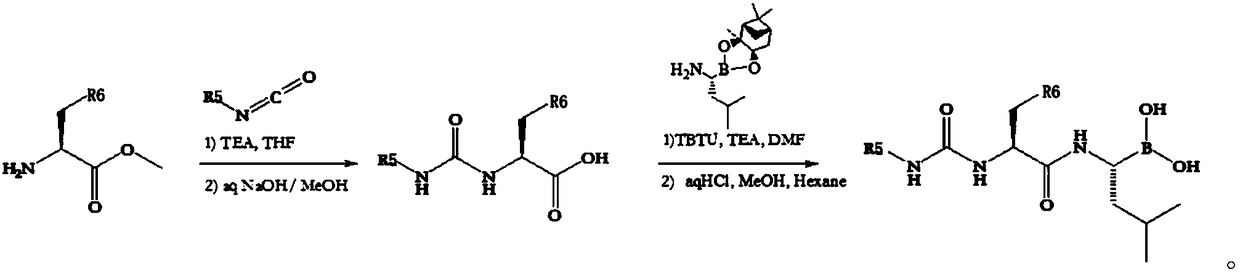
A class of dipeptide boronic acid compound and its preparation method and use
A dipeptide boronic acid and compound technology, applied in the field of dipeptide boronic acid compounds, can solve problems such as toxic and side effects, and achieve high activity, significant effect, and the effect of inhibiting proteasome
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Methyl 3-aminobenzoate (1.35g, 7.35mmol), pyridine-3-carboxylic acid (1.0g, 7.35mmol) and TBTU (2.86g, 22.35mmol) were put into a 50mL round bottom flask, and DMF (18mL) was added to dissolve Clear, cool down to 0°C in an ice-water bath, add DIPEA (2.88g, 8.94mL) dropwise, after the addition is complete, stir at room temperature overnight, dilute with ethyl acetate (20mL), and successively add 1mol / L hydrochloric acid (10mL) and saturated sodium bicarbonate The solution (10 mL) was washed, dried over anhydrous sodium sulfate, filtered, and concentrated to obtain 1.3 g of brown oil.
[0049] Put the brown oil and sodium hydroxide (1g, 25mmol) into a 50mL original bottom flask, add methanol (25mL) to dissolve, stir at room temperature overnight, spin to dry the solvent, and dissolve the residue in water (20mL) for clarification. Adjust the pH to 5 with hydrochloric acid, and filter with suction to obtain 1.1 g of a white intermediate solid, with a yield of 91.2%.
[0050] ...
Embodiment 2
[0057] The difference between this example and Example 1 is that the pyridine-3-carboxylic acid in Example 1 was replaced with benzoic acid, the obtained brown oil was 1.5g, and then the obtained white intermediate solid was 1.1g. The rate is 91.2%.
[0058] The structure of the white intermediate solid is:
[0059] The yellow intermediate solid was 0.39 g, and the yield was 65%. The structure of the yellow intermediate solid is:
[0060] The finished product of white solid is 80 mg, and the yield is 33%. The white solid finished product is the finished product 2.
[0061] The structure of finished product 2 is: The spectrum detection results of this structure are as follows:
[0062] MS m / z 337 (M-OH); 1 H-NMR (400MHz, DMSO) δ(ppm): 10.47~10.51(d, 1H), 7.96~8.06(m, 2H), 7.70~7.78(m, 1H), 7.78~7.61(m, 3H), 1.76 ~1.79(t, 1H), 1.40~1.45(m, 2H), 1.17~1.23(m, 1H), 0.57~0.83(t, 6H).
Embodiment 3
[0064] The difference between this example and Example 1 is that the pyridine-3-carboxylic acid (1.0g, 7.35mmol) in Example 1 was replaced by 2-furancarboxylic acid (0.98g, 8.8mmol), and the brown oil obtained was 1.5g, put the brown oil and sodium hydroxide (1g, 25mmol) into a 50mL original bottom flask, add methanol (25mL) to dissolve, stir at room temperature overnight, spin to dry the solvent, and dissolve the residue with water (20mL). Adjust the pH to 5 with 2 mol / L hydrochloric acid, and filter with suction to obtain 0.98 g of white intermediate solid, with a yield of 83.2%.
[0065] The structure of the white intermediate solid is:
[0066] Put the above-mentioned white intermediate solid (0.34g, 1.45mmol), raw material three (0.5g, 1.32mmol) and TBTU (0.51g, 1.58mmol) into a 50mL round-bottomed flask, add DMF (4mL) to dissolve, and cool to At 0°C, DIPEA (2.88g, 8.94mL) was added dropwise. After the addition was complete, the mixture was stirred at 0°C for 5min, the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com