Application of lncRNA-MALAT1 in preparing proliferative vitroretinopathy (PVR) diagnosis reagent
A vitreoretinal and diagnostic reagent technology, applied in the field of medical biological detection, can solve problems such as proliferative vitreoretinopathy without lncRNA-MALAT1, and achieve the effect of less trauma and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
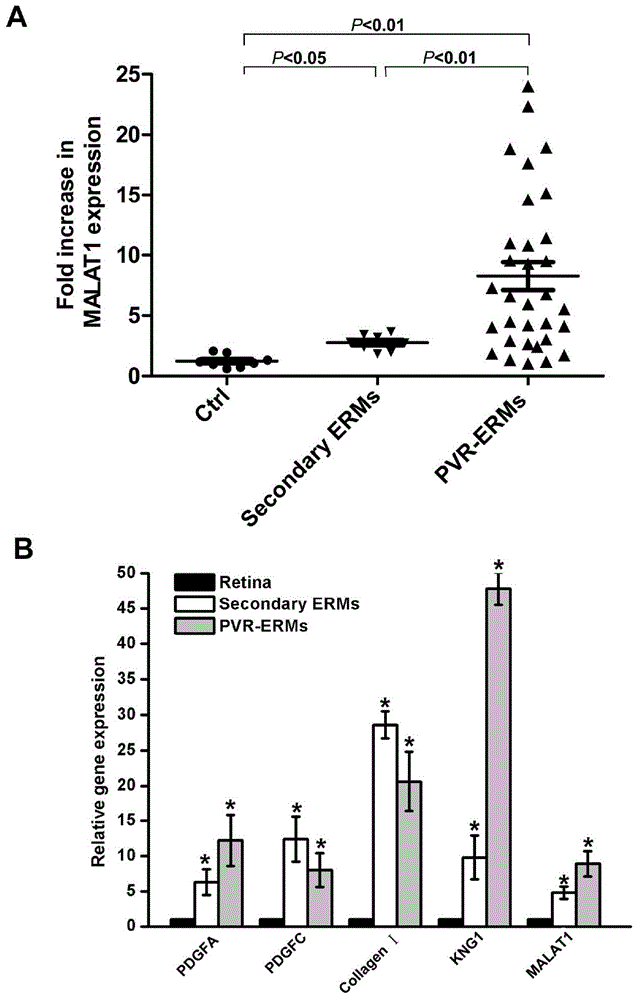
[0062] Example 1 Verification of the correlation between lncRNA-MALAT1 and proliferative vitreoretinopathy
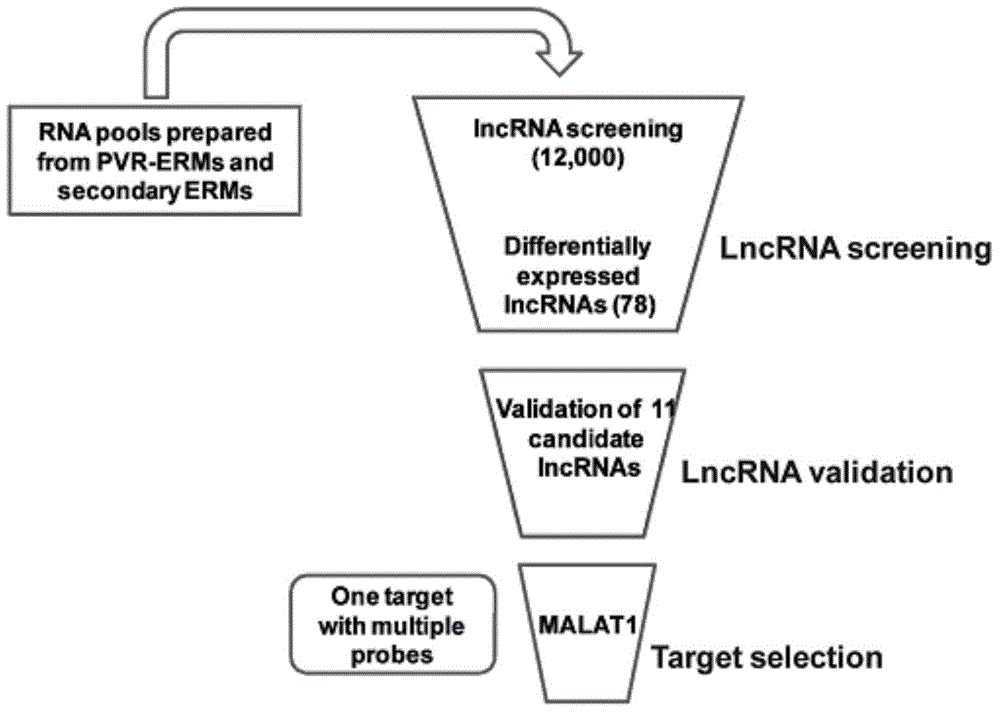
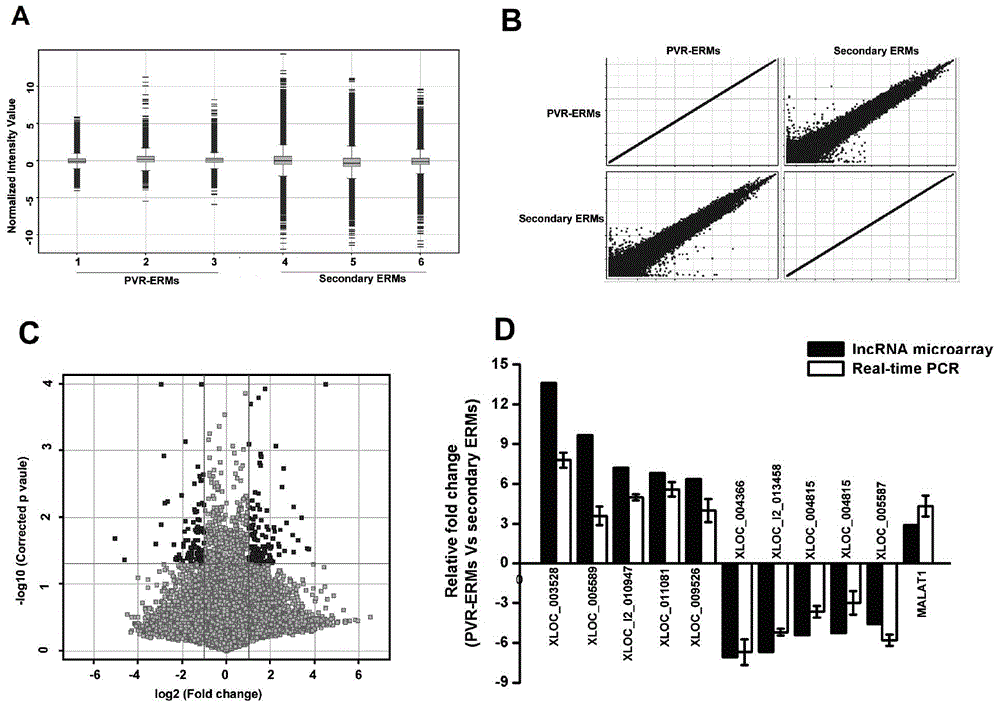
[0063] (1) lncRNA microarray analysis and screening of lncRNA related to PVR disease and verification, the specific process is as follows figure 1 shown.
[0064] The first step: sample preparation: epiretinal membrane specimens (experimental group, n=30) and cataract proliferation membrane specimens (control group, n=30) after ophthalmic vitreous surgery, total RNA was extracted with TRIzol (Invitrogen) reagent, and preserved Store at -80°C for later use.
[0065] Step 2: Differentially expressed lncRNA screening:
[0066] Using the lncRNA expression profile chip of Aglient Company in the United States, the lncRNA related to the occurrence of PVR disease was analyzed; the specific steps of the analysis were as follows: the fluorescent group was used to label the lncRNA with a labeling enzyme to obtain a fluorescent probe for hybridization with the chip, and MAUI was ...
Embodiment 2
[0071] Embodiment 2: preparation kit of the present invention
[0072] The sequence of MALAT1 is shown in SEQ ID: NO: 1, its specific quantitative PCR upstream and downstream primers and internal reference GAPDH and / or Beta-tubulin quantitative PCR upstream and downstream primers, designed by Primer5, is responsible for primer synthesis by Invitrogen Company, the purity is PAGE grade, synthesized primers using DEPC H 2 O was dissolved to a total concentration of 10 μM.
[0073] Prepare a kit comprising the following components:
[0074] (a) Extraction system
[0075] 1) Trizol reagent, 1 tube, 2000 μL / tube;
[0076] 2) Chloroform, 1 tube, 500 μL / tube;
[0077] 3) Absolute ethanol, 1 tube, 8000 μL / tube;
[0078] 4) DEPC ddH2O, 1 tube, 1000 μL / tube;
[0079] 5) ddH2O, 1 tube, 2000 μL / tube;
[0080] 6) Isopropanol, 8000 μL / tube;
[0081] (b) Reverse transcription system
[0082]1) Total RNA reverse transcription primers (including Oligo dT and Random6mers), 1 tube, conce...
Embodiment 3
[0096] Embodiment 3: kit detection of the present invention
[0097] 1. Separation of plasma and blood cell samples
[0098] The blood samples of the examined individuals and their reference healthy individuals were collected, and serum and blood cells were separated by centrifugation using heparin anticoagulant tubes for the detection of lncRNA. The centrifugation conditions were 4°C, 12,000 rpm, 10 min.
[0099] 2. RNA Extraction from Plasma and Blood Cell Samples
[0100] In the separated blood cells and plasma samples, use the total RNA extraction system of the kit of the present invention, add TRIzol and place it at room temperature for 10 minutes to fully lyse the sample (note: if the next step is not performed, the sample can be placed in- long-term storage at 70°C). Add 200 μl of chloroform to every 1ml of TRIzol, vibrate vigorously and mix well, then place at room temperature for 3-5min to allow natural phase separation. Centrifuge at 12,000 rpm at 4°C for 15 min....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com