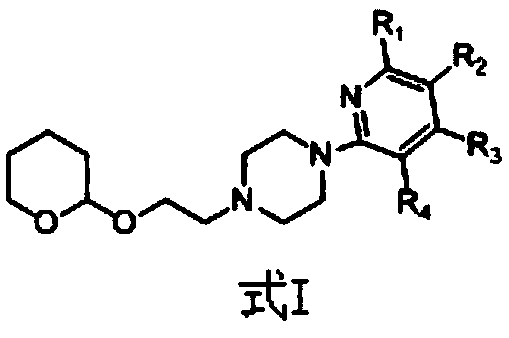
Derivative containing nitrogen heterocyclic rings and application of derivative in retinal neovascularization diseases
A technology of new blood vessels and derivatives, applied in the field of medicinal chemistry and drug activity testing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
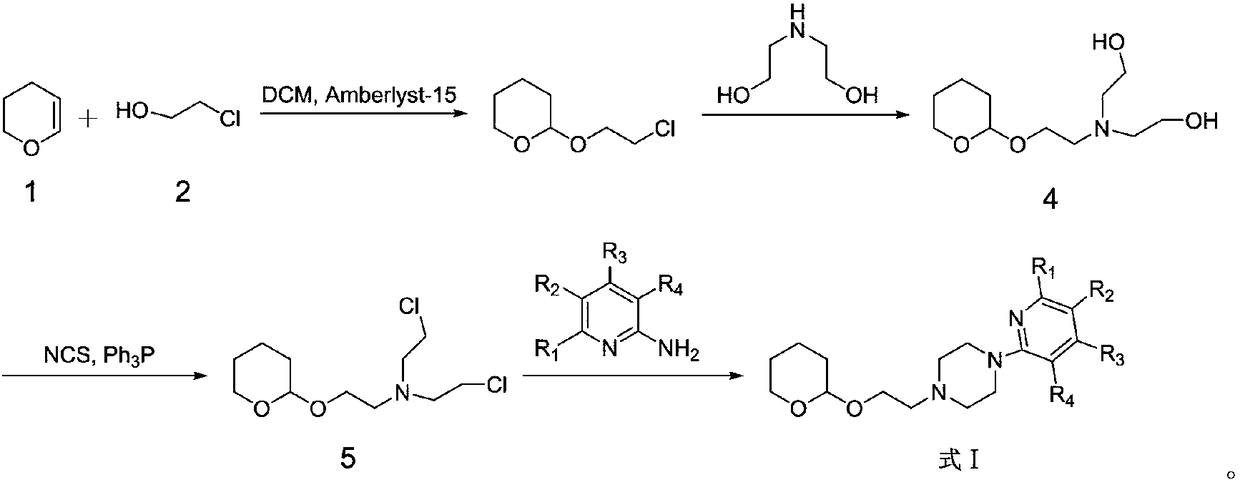
[0021] Example 1: Synthesis of 1-(pyridin-2-yl)-4-(2-((tetrahydro-2H-pyran-2-yl)oxy)ethyl)piperazine
[0022] 1. Synthesis of 2-(2-chloroethoxy)tetrahydro-2H-pyran:
[0023]
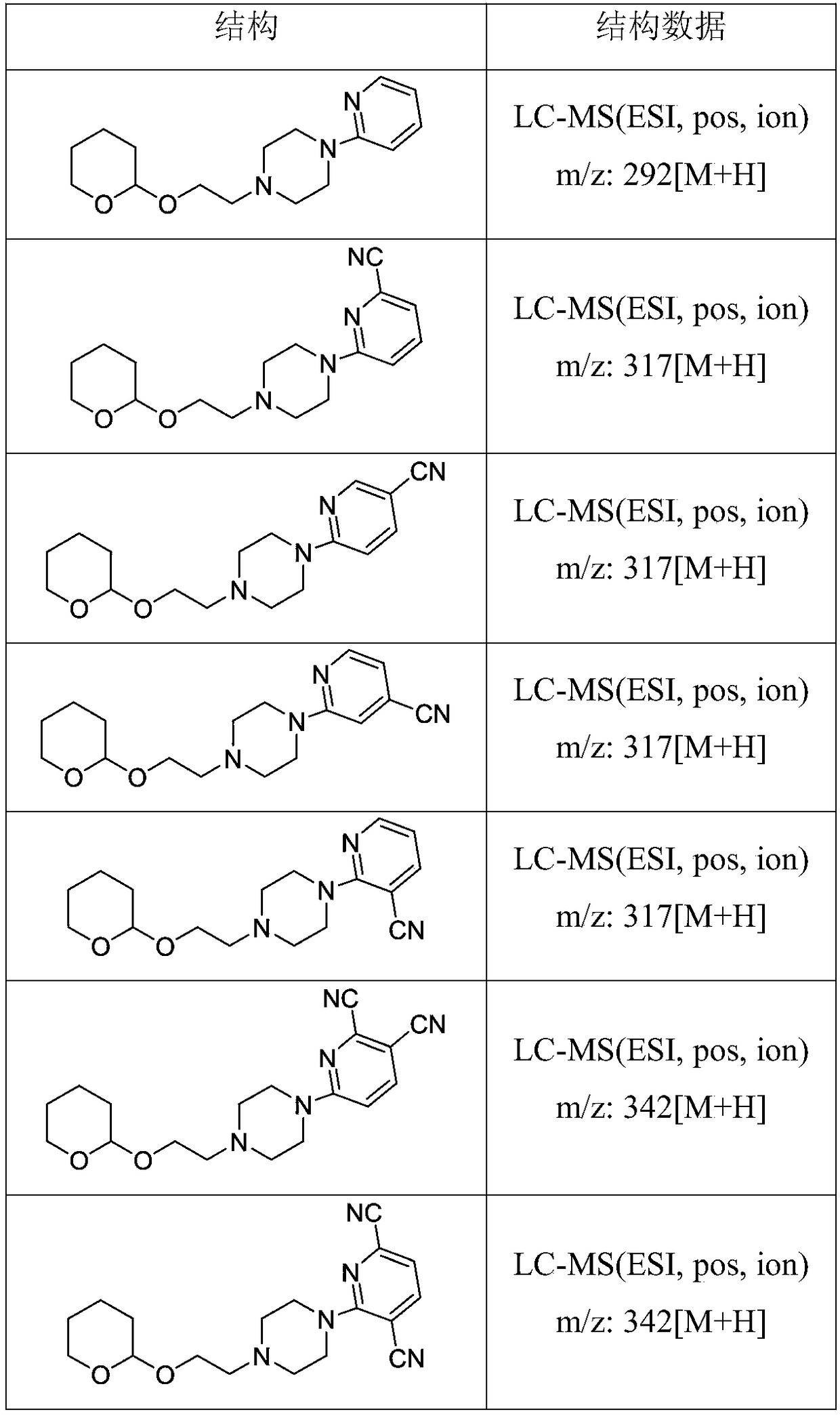
[0024] 2-Chloro-1-ethanol (21.74 g, 0.27 mol), dichloromethane (50 mL) and Amberlyst H-15 (1 g) were added to the reaction flask together, followed by 3,4-dihydro -2H-pyran (25 mL, 0.27 mol), the reaction mixture was stirred at 25°C for 17 hours. After filtering off Amberlyst H-15, dichloromethane was evaporated in vacuo to give 2-(2-chloroethoxy)tetrahydro-2H-pyran (compound 3), 39.12g. The yield was 88%. It was directly used in the next step without purification. 1 H-NMR (400MHz, CDCl3) δ: 1.46-1.84 (m, 6H), 3.59-3.79 (m, 6H), 4.52 (t, 1H). 13 C-NMR (125MHz, CDCl3) δ: 19.74, 25.57, 30.21, 42.73, 65.40, 65.98, 99.57. LC-MS (ESI, pos, ion) m / z: 166[M+H].
[0025] 2. Synthesis of 2,2'-((2-((tetrahydro-2H-pyran-2-yl)oxy)ethyl)amino)bis(ethane-1-ol):
[0026]
[0027] Add diethanolamine (25.23g,...
Embodiment 2
[0034] Example 2: Synthesis of 1-(6-cyano-pyridin-2-yl)-4-(2-((tetrahydro-2H-pyran-2-yl)oxy)ethyl)piperazine
[0035]
[0036] Compound 5 (52.90g, 0.20mol), 6-cyano-pyridin-2-amine (0.21mol) and N,N-dimethylformamide (DMF) (30mL) were added to the reaction flask together, and then 60% sodium hydride (5.2 g, 0.13 mol) was added in portions. The reaction mixture was stirred at 50°C for 40 minutes, then warmed to 105°C and stirred for 4.5 hours. It was then cooled to 20°C and water (30 mL) was added. The product was extracted with ethyl acetate (50 mL), then washed with water (20 mL), the solvent was concentrated under reduced pressure, and flash column chromatography was used to obtain 54.42 g of off-white solid 1-(6-cyano-pyridin-2-yl )-4-(2-((tetrahydro-2H-pyran-2-yl)oxy)ethyl)piperazine in 86% yield. LC-MS (ESI, pos, ion) m / z: 317 [M+H].
Embodiment 3
[0037] Example Three: Synthesis of 1-(5-cyano-pyridin-2-yl)-4-(2-((tetrahydro-2H-pyran-2-yl)oxy)ethyl)piperazine
[0038]
[0039] Compound 5 (52.90g, 0.20mol), 5-cyano-pyridin-2-amine (0.21mol) and N,N-dimethylformamide (DMF) (30mL) were added to the reaction flask together, and then 60% sodium hydride (5.2 g, 0.13 mol) was added in portions. The reaction mixture was stirred at 50°C for 40 minutes, then warmed to 105°C and stirred for 4.5 hours. It was then cooled to 20°C and water (30 mL) was added. The product was extracted with ethyl acetate (50 mL), then washed with water (20 mL), the solvent was concentrated under reduced pressure, and flash column chromatography was used to obtain 51.89 g of off-white solid 1-(5-cyano-pyridin-2-yl )-4-(2-((tetrahydro-2H-pyran-2-yl)oxy)ethyl)piperazine in 82% yield. LC-MS (ESI, pos, ion) m / z: 317 [M+H].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com