Connective Tissue Growth Factor Fragments and Methods and Uses Thereof
a technology of connective tissue and growth factor, which is applied in the field of growth factor fragments, can solve the problems that ctgf alone cannot induce this property in fibroblasts, and achieve the effects of suppressing, increasing the activity of ctgf fragments, and inhibiting or suppressing the activity of ctg
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
CTGF Fragments Stimulate Extracellular Matrix Synthesis
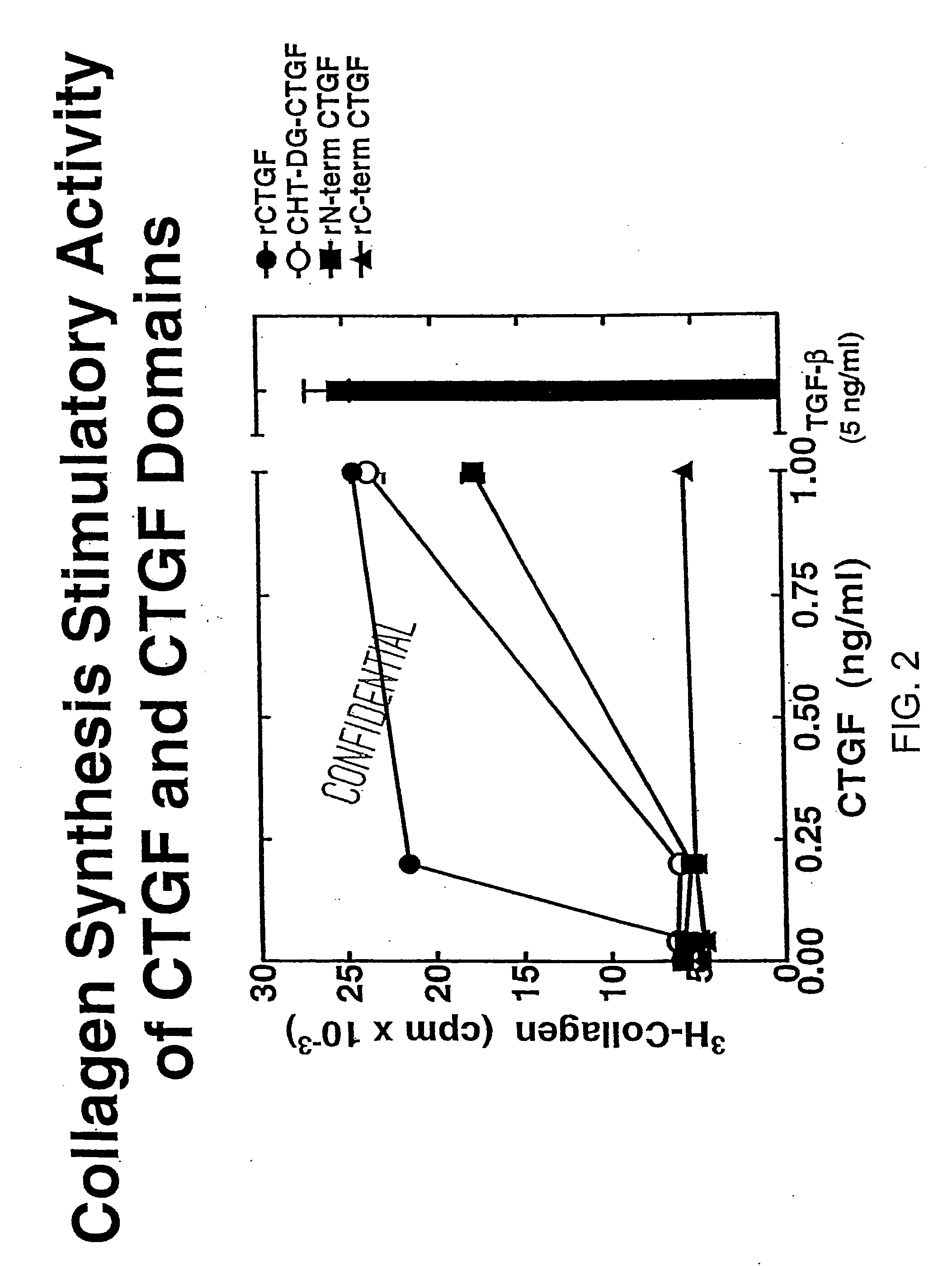
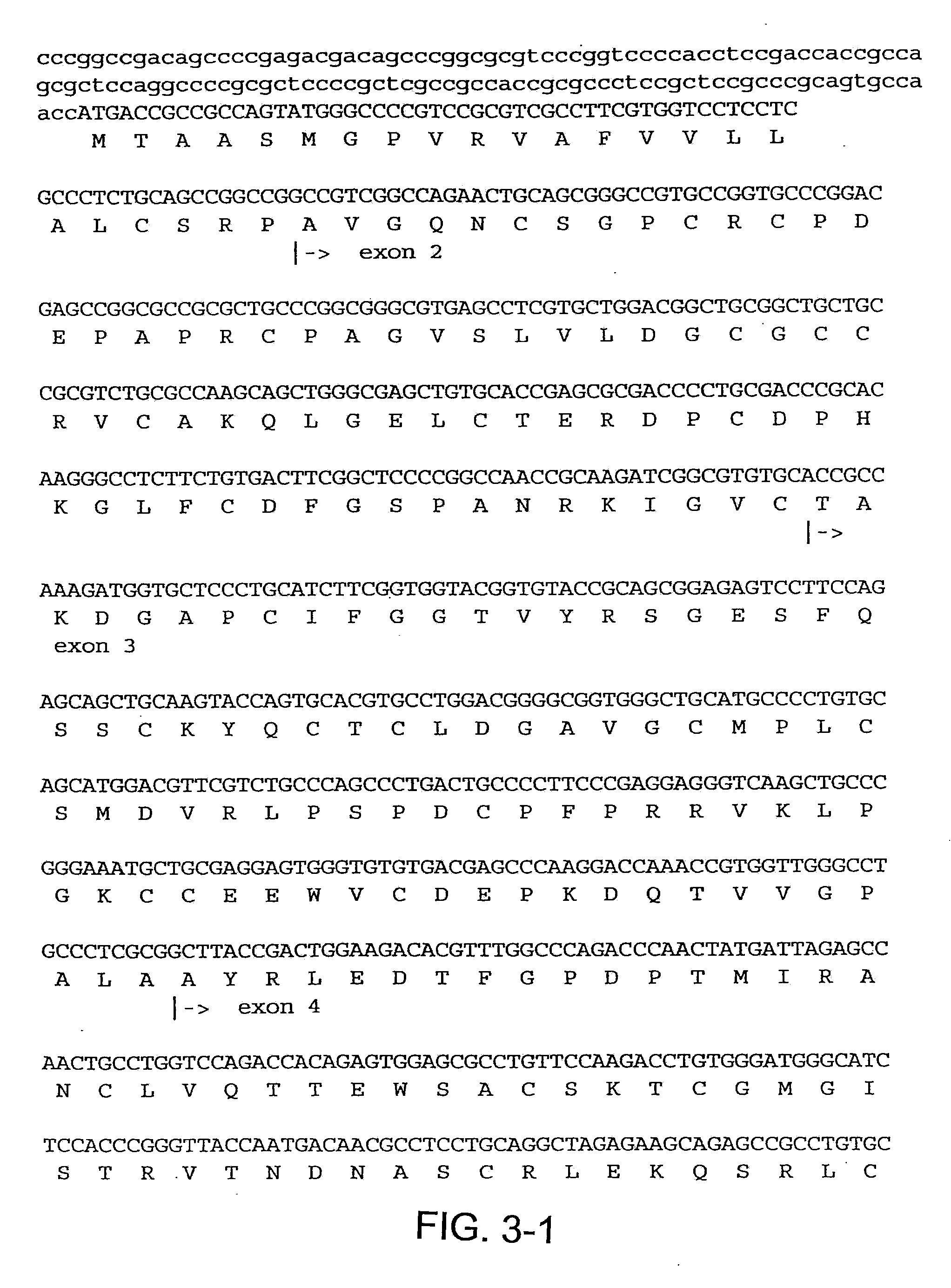
[0157]To prepare CTGF fragments, human recombinant CTGF (full length) was digested by chymotrypsin to render one CTGF fragment. Recombinant CTGF fragments were also produced by expressing either or both exon 2 and exon 3 of CTGF. A continuous line of cultured normal rat kidney (NRK) fibroblasts, designated as clone NRK-49F, were obtained from the American Type Culture Collection (ATCC) to produce cell cultures. Human foreskin fibroblasts were established from explant cultures. Cell cultures were maintained in Dulbecco's modified eagle media (DME) containing 2.5% fetal bovine serum and 2.5% Nu-Serum I (Collaborative Biomedical Products, Bedford, Mass.) and passaged prior to confluence.
[0158]To examine fibroblast collagen synthesis, growth-arrested monolayers of NRK and human foreskin fibroblasts were prepared by seeding 10,000 cells / well in 48 well plates and allowing the cells to grow to confluence in 5 to 7 days in DME and 2.5%...
example 2
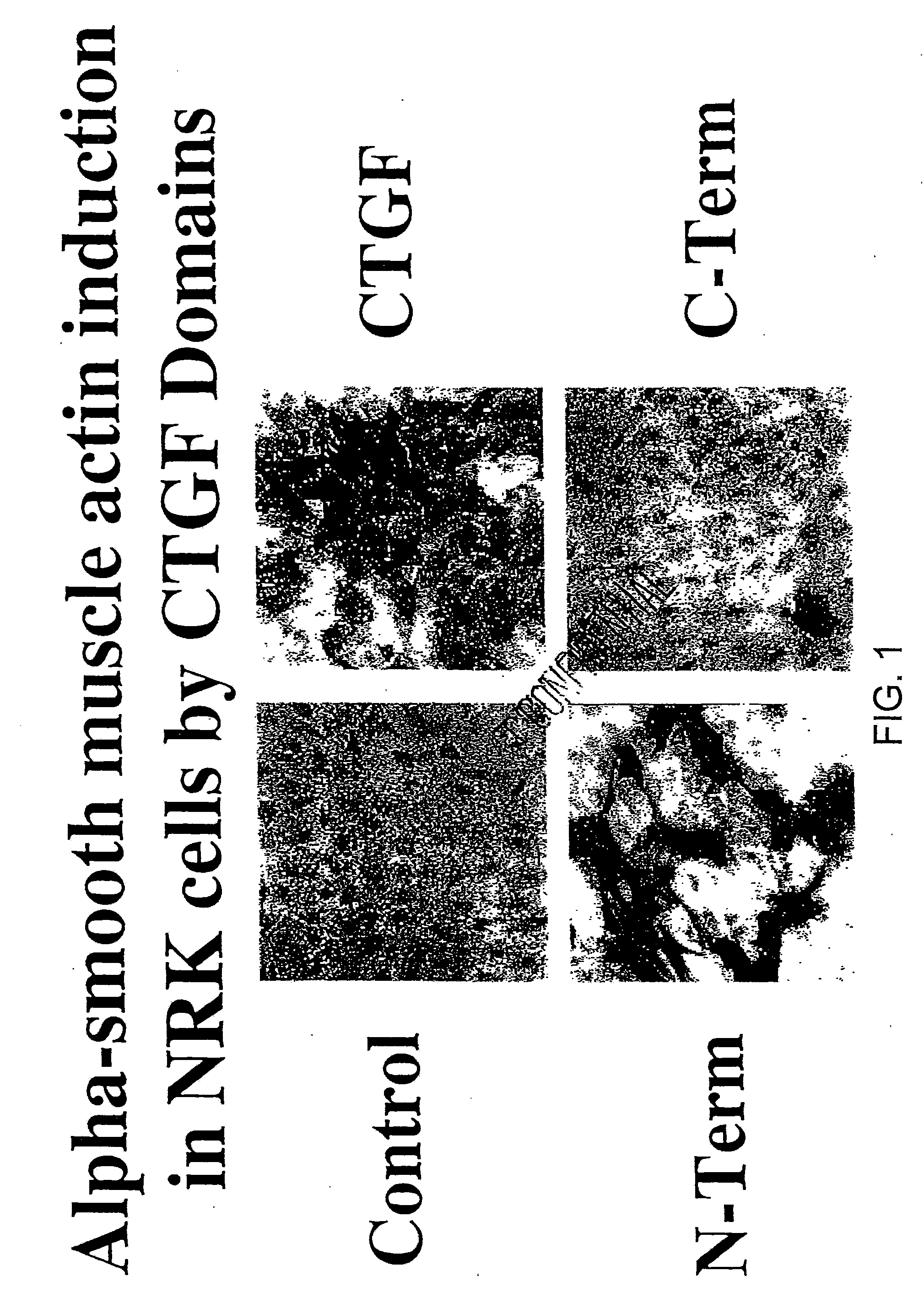
CTGF Fragments Induce Myofibroblast Differentiation
[0159]The CTGF fragments and cell cultures were prepared as described above. To examine induction of myofibroblasts by CTGF fragments, growth-arrested monolayers of NRK for foreskin fibroblasts were prepared and treated as described above. Following treatments, 48-well plate monolayers were washed twice with TBS and fixed in methanol at −20 degrees C. for 10 minutes before processing for immunohistological detection of alpha-smooth muscle. Immunohistological detection was conducted. Following fixation, cell monolayers were washed twice with TBS and blocked for 30 minutes with 10% horse serum / 2% milk in TBS. The cells were then incubated for 1 hour with monoclonal mouse anti alpha-smooth muscle actin IgG (Clone 1A4, Sigma Chemical) at a 1:200 dilution in horse serum / milk / TBS. Following three washes with TBS, cell monolayers were then incubated for 1 hour with biotinlyated horse anti-mouse IgG (Vector Labs, Burlingame, Calif.) at a 1:...
example 3
Neutralizing Anti-CTGF Antibodies Block TGF-B Induced DNA Synthesis, Collagen Synthesis, and Myofibroblast Induction
[0161]Specific anti-CTGF antibodies were raised against biologically active recombinant human CTGF produced in a baculovirus expression system using methods known in the art. The antibodies were prepared in goats and tested for neutralization activity of CTGF directly or on TGF-induced DNA or collagen synthesis in NRK fibroblasts. The goat antibodies exhibited activity in the assays for neutralization of TGF-13 action. In these assays, the goat anti-CTGF antibodies were able to block DNA synthesis. In addition, as demonstrated in FIG. 5, CTGF antibodies were able to block collagen synthesis and myofibroblast formation induced by TGF-β. It was noted that the amount of antibody required to block collagen synthesis was significantly less than the amount needed to block DNA synthesis. Both western Mot assay, and competition ELISA assays indicated that most of the antibodie...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| Concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com