Patents
Literature
47 results about "Soft agar" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
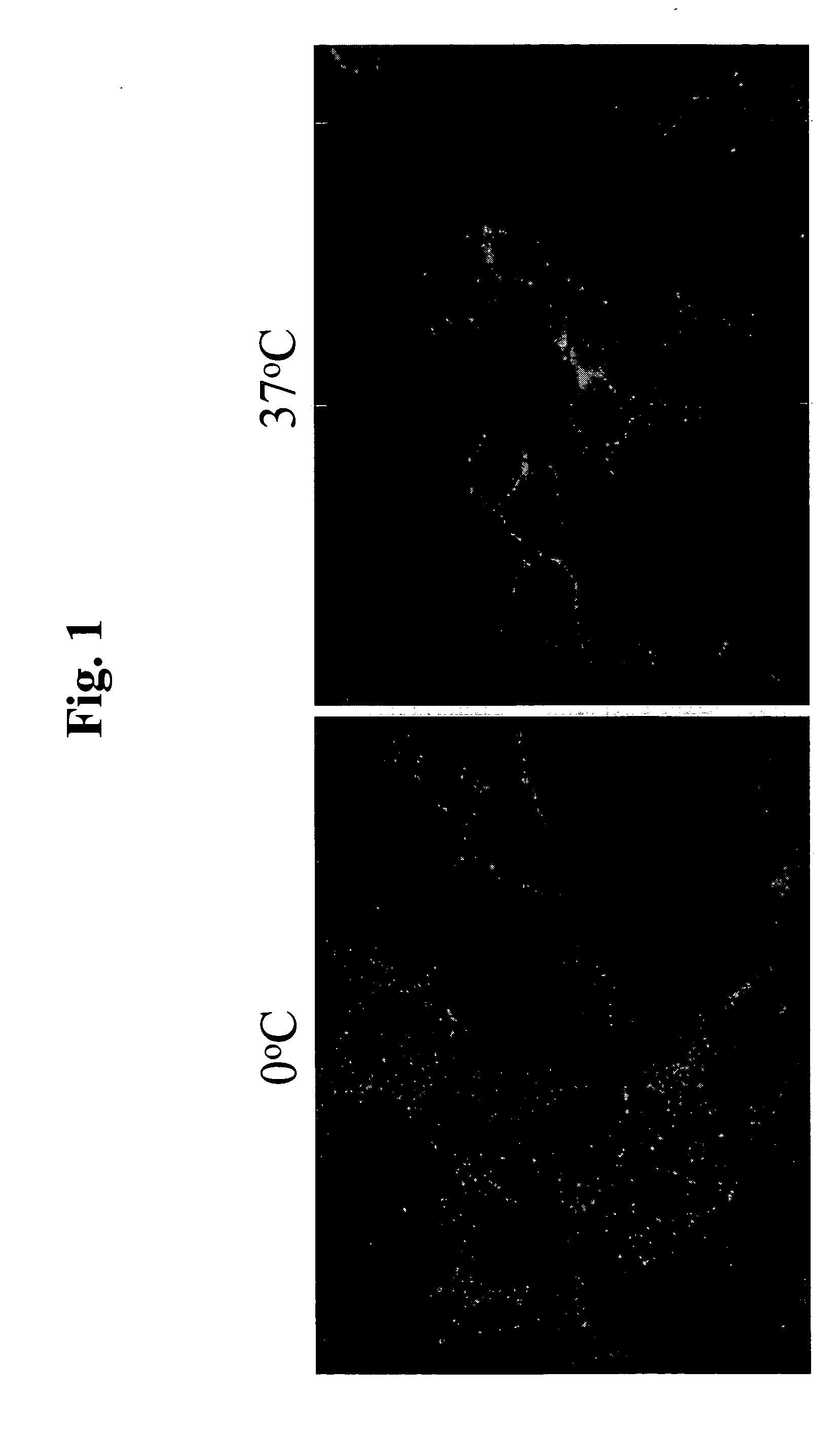
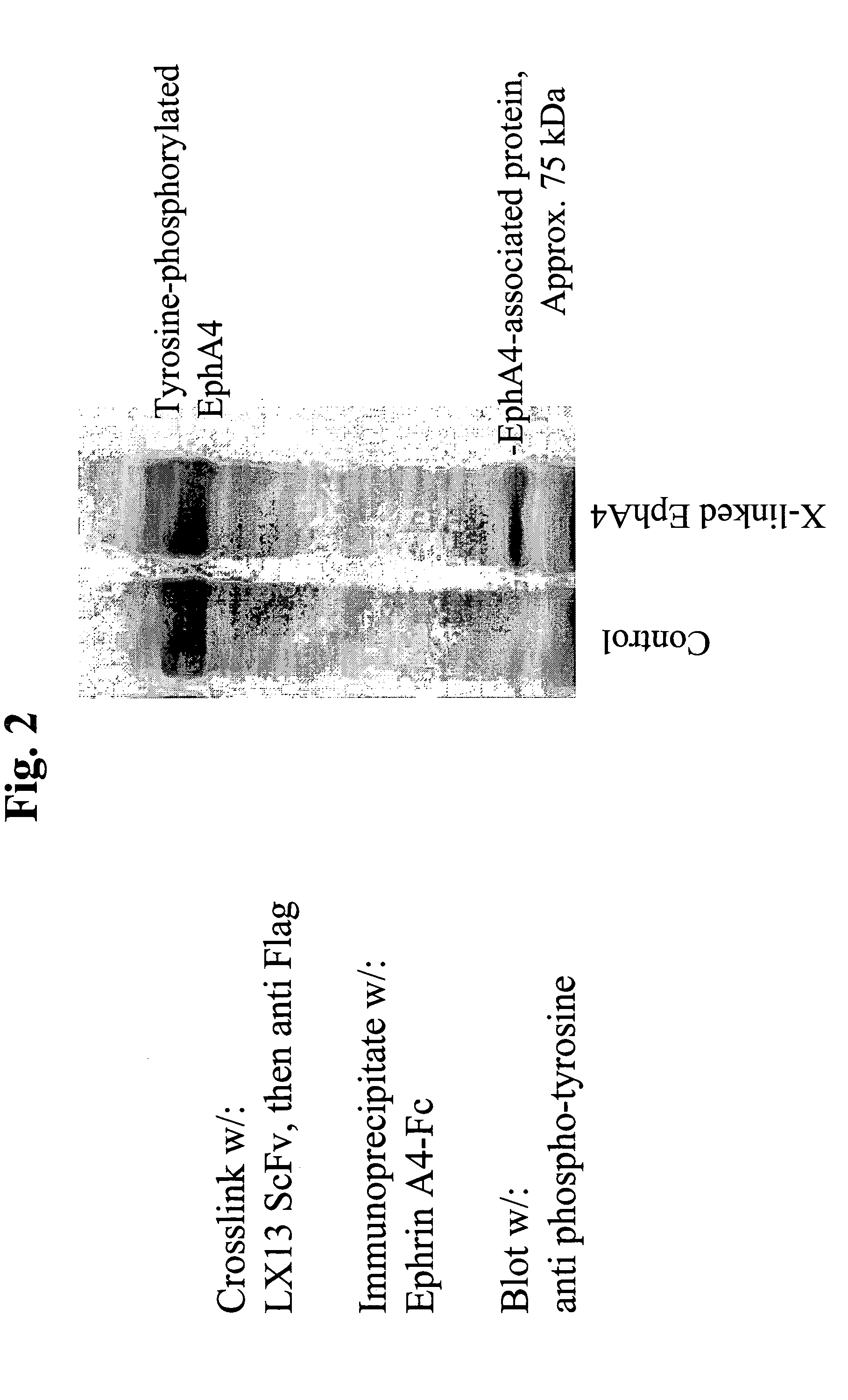
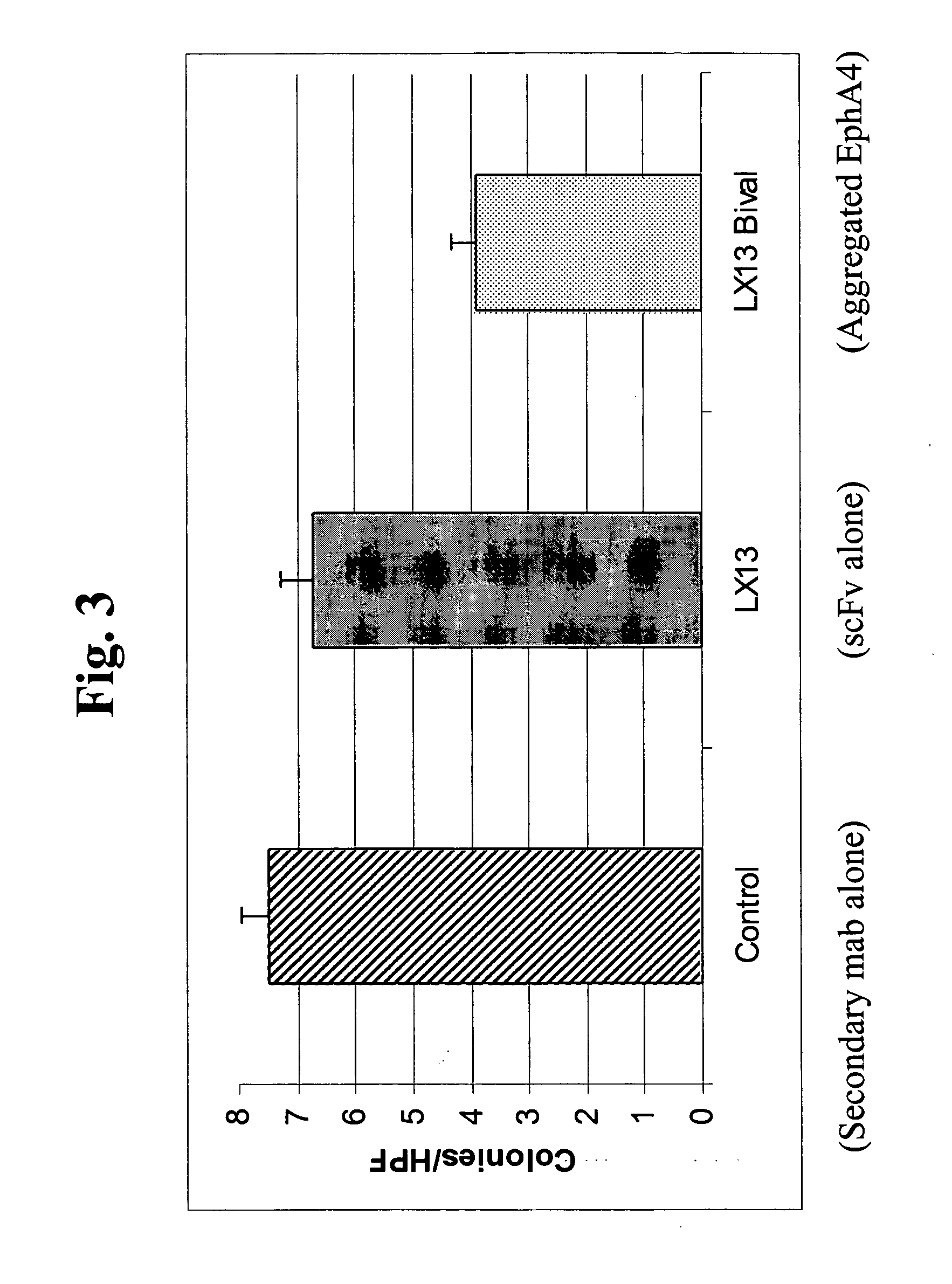
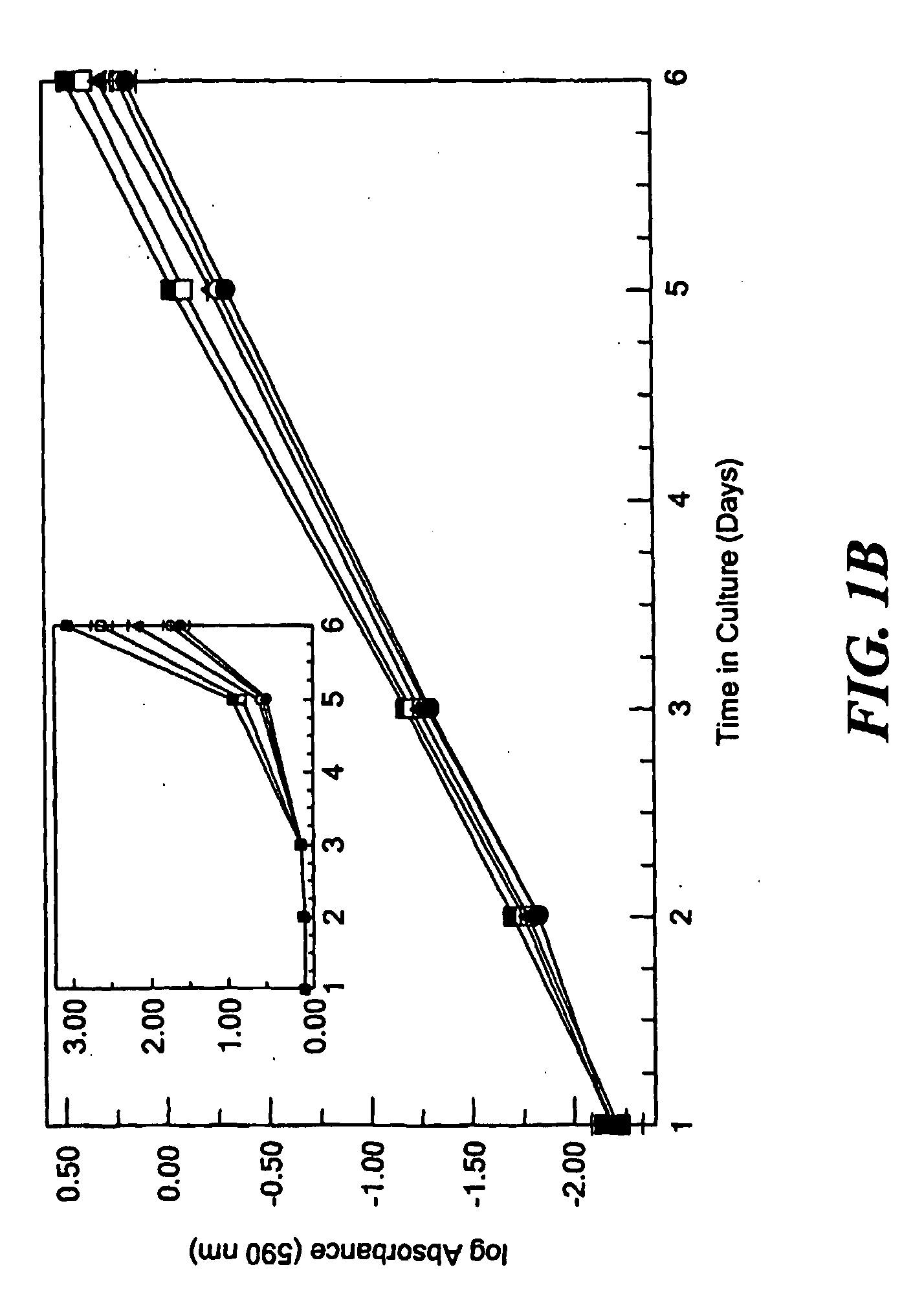
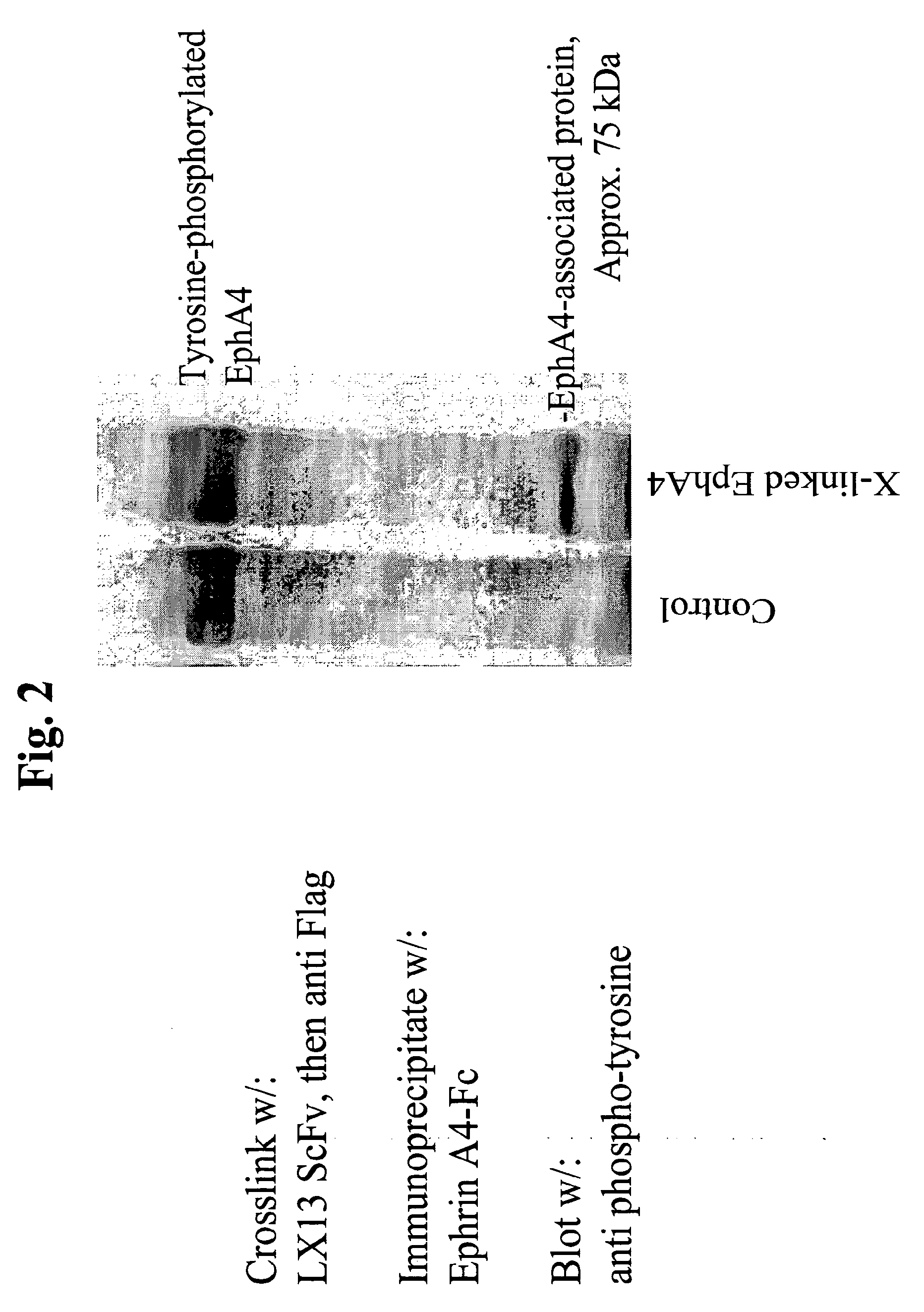
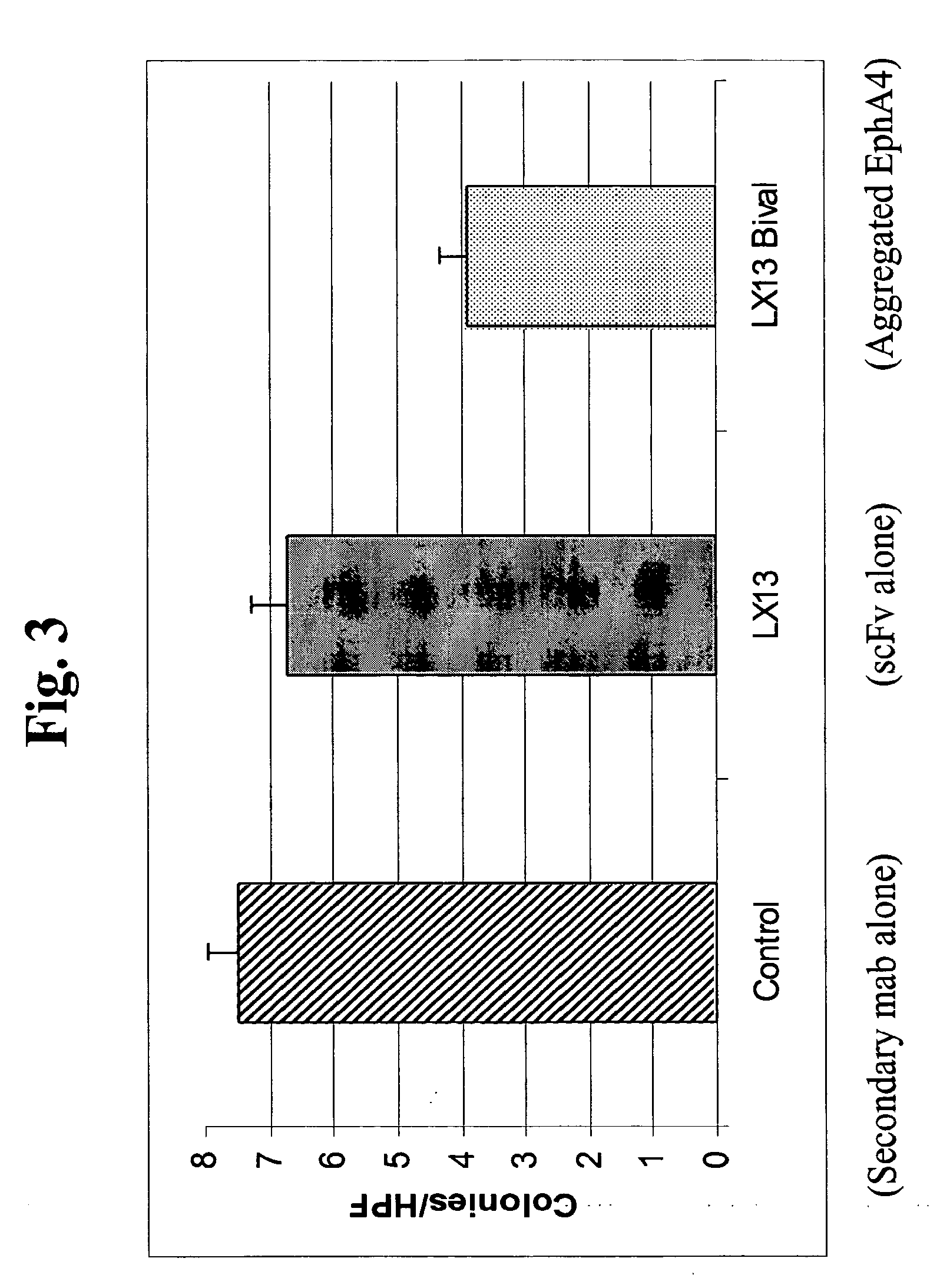
Use of EphA4 and modulator of EphA4 for diagnosis, treatment and prevention of cancer
InactiveUS20050013819A1Reduce spreadInhibit tumor cell growthAntibody mimetics/scaffoldsImmunoglobulins against cytokines/lymphokines/interferonsCancer preventionNon cancer
The present invention relates to methods and compositions designed for the treatment, management, or prevention of cancer, particularly, metastatic cancer. In one embodiment, the methods of the invention comprise the administration of an effective amount of one or more antibodies that bind to EphA4 and agonize EphA4. In another embodiment, the methods of the invention comprise the administration of an effective amount of one or more antibodies that bind to EphA4 and inhibit cancer cell colony formation in soft agar or tubular network formation in three-dimensional basement membrane or extracellular matrix preparation. In another embodiment, the methods of the invention comprise the administration of an effective amount of one or more antibodies that preferentially binds to an EphA4 epitope that is exposed on cancer cells but not non-cancer cells. In another embodiment, the methods of the invention comprise the administration of an effective amount of one or more antibodies that bind to EphA4 with a very low Koff to reduce EphA4 expression and, thereby, inhibit tumor cell growth and / or metastasis. The invention also provides pharmaceutical compositions comprising one or more EphA4 antibodies of the invention either alone or in combination with one or more other agents useful for cancer therapy.
Owner:MEDIMMUNE LLC
Methods and compositions for diagnosis and treatment of cancer
The present invention relates to a novel gene, CaSm, that is highly expressed in cancer tissues and cell lines, especially pancreatic cancer. The full length cDNA of CaSm encodes a protein of 133 amino acids. CaSm contains the two Sm motifs found in the common snRNP proteins, with the greatest homology to the Sm G protein (60% similarity). The present invention further encompasses CaSm peptides, fusion proteins, host cell expression systems, antibodies to CaSm, antisense CaSM molecules, and compounds that modulate CaSm gene expression or CaSm activity. Antisense CaSm RNA is able to alter the transformed phenotype of pancreatic cancer cells by reducing their ability to form large colonies in soft agar when compared to untransfected cells. The present invention also encompasses methods for disease diagnosis, drug screening and the treatment of cancer.
Owner:MUSC FOUND FOR RES DEV
Use of the Pro-Peptide Domain of Lysyl Oxidase as a Therapeutic Agent
A therapeutic composition that includes an active portion of the lysyl oxidase pro-peptide in a pharmaceutically acceptable carrier substance and methods of using such a therapeutic composition are disclosed. The active agent does not have lysyl oxidase enzymatic activity. Preferably, the active polypeptide is active in inhibiting cell growth in soft agar and active in inhibiting tumor formation. In addition, the active polypeptide preferably comprises an active portion of the amino acid sequence given in SEQ ID NO.: 1 or SEQ ID NO.: 2, or conservative substitions thereof. Alternatively, the active polypeptide comprises a polypeptide comprising an active portion of an amino acid sequence selected from the group consisting of SEQ ID NOs.: 3-8, or conservative substitions thereof.
Owner:TRACKMAN PHILIP C +3
USE OF EphA4 AND MODULATOR OF EphA4 FOR DIAGNOSIS, TREATMENT AND PREVENTION OF CANCER
InactiveUS20100166657A1Promote growthReduce expressionAntibody mimetics/scaffoldsImmunoglobulins against cytokines/lymphokines/interferonsCancer preventionCancer cell
The present invention relates to methods and compositions designed for the treatment, management, or prevention of cancer, particularly, metastatic cancer. In one embodiment, the methods of the invention comprise the administration of an effective amount of one or more antibodies that bind to EphA4 and agonize EphA4. In another embodiment, the methods of the invention comprise the administration of an effective amount of one or more antibodies that bind to EphA4 and inhibit cancer cell colony formation in soft agar or tubular network formation in three-dimensional basement membrane or extracellular matrix preparation. In another embodiment, the methods of the invention comprise the administration of an effective amount of one or more antibodies that preferentially binds to an EphA4 epitope that is exposed on cancer cells but not non-cancer cells. In another embodiment, the methods of the invention comprise the administration of an effective amount of one or more antibodies that bind to EphA4 with a very low Koff to reduce EphA4 expression and, thereby, inhibit tumor cell growth and / or metastasis. The invention also provides pharmaceutical compositions comprising one or more EphA4 antibodies of the invention either alone or in combination with one or more other agents useful for cancer therapy.
Owner:MEDIMMUNE LLC
EpA2 monoclonal antibodies and methods of use thereof
InactiveUS20070166314A1Lower Level RequirementsIncreased phosphorylationOrganic active ingredientsFungiNon cancerCancer prevention
The present invention relates to methods and compositions designed for the treatment, management, or prevention of cancer, particularly, metastatic cancer. In one embodiment, the methods of the invention comprise the administration of an effective amount of an antibody that binds to EphA2 and agonizes EphA2, thereby increasing EphA2 phosphorylation and decreasing EphA2 levels. In other embodiments, the methods of the invention comprise the administration of an effective amount of an antibody that binds to EphA2 and inhibits cancer cell colony formation in soft agar, inhibits tubular network formation in three-dimensional basement membrane or extracellular matrix preparation, preferentially binds to an EphA2 epitope that is exposed on cancer cells but not non-cancer cells, and / or has a low Koff, thereby, inhibiting tumor cell growth and / or metastasis. The invention also provides pharmaceutical compositions comprising one or more EphA2 antibodies of the invention either alone or in combination with one or more other agents useful for cancer therapy.
Owner:MEDIMMUNE LLC
Epha2 monoclonal antibodies and methods of use thereof
InactiveUS20100278838A1Lower Level RequirementsReduce proliferationOrganic active ingredientsFungiCancer preventionAntiendomysial antibodies
The present invention relates to methods and compositions designed for the treatment, management, or prevention of cancer, particularly, metastatic cancer. In one embodiment, the methods of the invention comprise the administration of an effective amount of an antibody that binds to EphA2 and agonizes EphA2, thereby increasing EphA2 phosphorylation and decreasing EphA2 levels. In other embodiments, the methods of the invention comprise the administration of an effective amount of an antibody that binds to EphA2 and inhibits cancer cell colony formation in soft agar, inhibits tubular network formation in three-dimensional basement membrane or extracellular matrix preparation, preferentially binds to an EphA2 epitope that is exposed on cancer cells but not non-cancer cells, and / or has a low Koff, thereby, inhibiting tumor cell growth and / or metastasis. The invention also provides pharmaceutical compositions comprising one or more EphA2 antibodies of the invention either alone or in combination with one or more other agents useful for cancer therapy.
Owner:MEDIMMUNE LLC
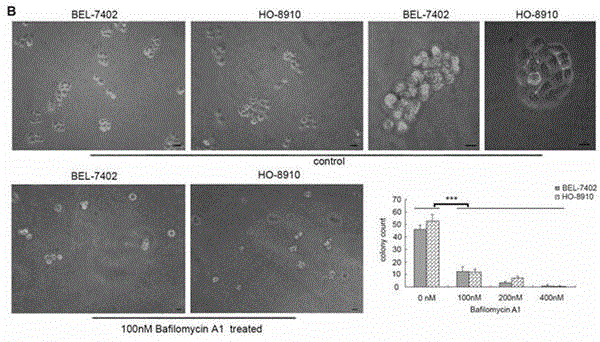
Micro RNA related to BafilomycinA1 liver cancer and ovarian cancer inhibition cell line
InactiveCN104630224AEliminate genetic differencesStrong specificityDNA/RNA fragmentationAffymetrix genechipControl cell
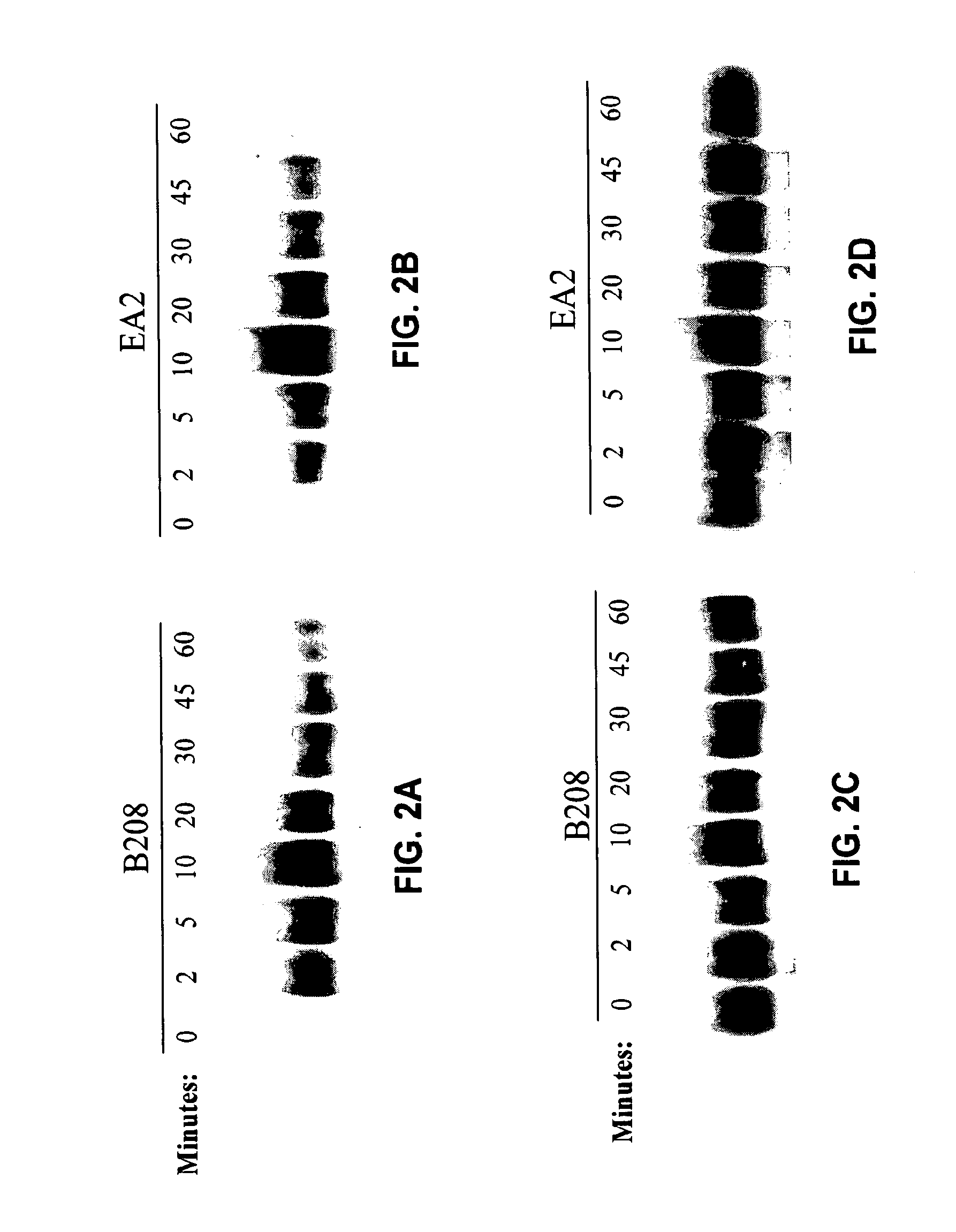
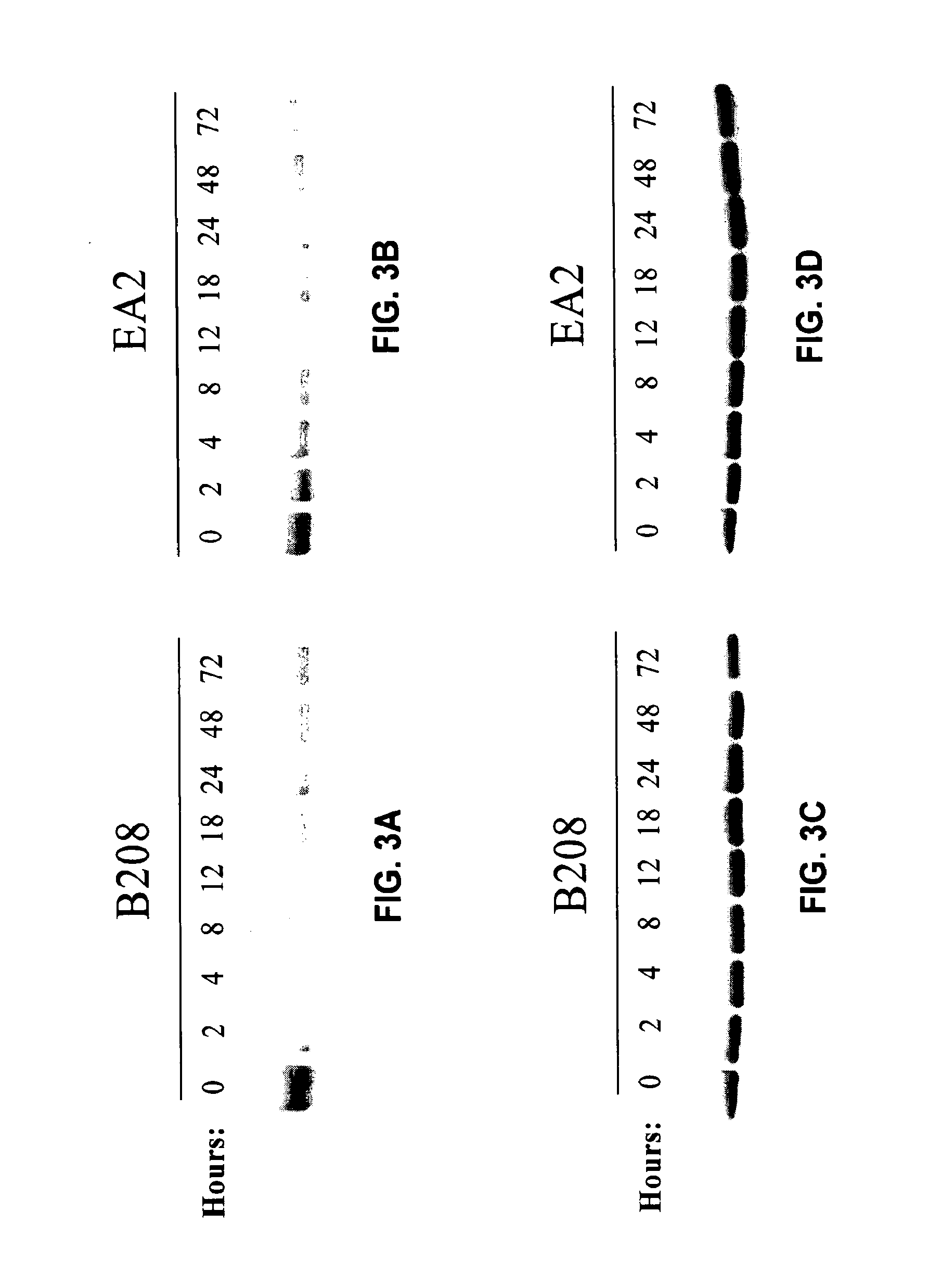
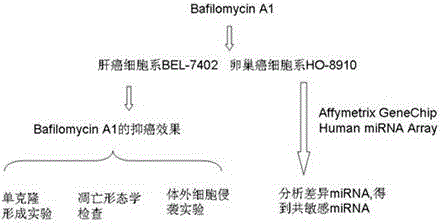
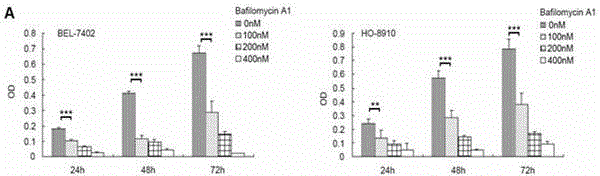
The invention relates to a group of micro RNA related to a BafilomycinA1 liver cancer and ovarian cancer inhibition cell line, belonging to the technical field of medical biology. The preparation method comprises the following steps: respectively acting BafilomycinA1 on a liver cancer cell line BEL-7402 and an ovarian cancer cell line HO-8910, and detecting the in-vitro growth inhibition, apoptosis promoting and invasion inhibition effects of BafilomycinA1 on two cells by performing soft agar cloning formation assay, electron microscopy observation, apoptosis related enzyme detection and in-vitro invasion assay; respectively acting 400nM BafilomycinA1 on the liver cancer cell line BEL-7402 and the ovarian cancer cell line HO-8910 for 48 hours, collecting dosing cells and control cells, extracting the RNA, analyzing the high-flux micro RNA between the drug group and the control group by using Affymetrix GeneChip Human Gene Array micro RNA array, and verifying the differences of the micro RNA by using qPCR, thereby obtaining co-sensitive micro RNA of the two cells. According to the group of micro RNA sensitive to the BafilomycinA1, on one hand, the micro RNA has the cancer inhibition effectiveness corresponding to the BafilomycinA1; on the other hand, the micro RNA has obvious change in two tumor cells, and a basis is provided for targets designed by taking the micro RNA as a potential broad spectrum anti-cancer drug.
Owner:JIANGSU UNIV
Preparation method of immortalized human intervertebral disc nucleus pulposus cell system
ActiveCN102093980AImprove the level of prevention and controlHave a normal growth rateEnzymesVector-based foreign material introductionTelomeraseReverse transcriptase
The invention discloses a preparation method of an immortalized human intervertebral disc nucleus pulposus cell system. The preparation method is as follows: extrinsic human telomerase reverse transcriptase is transfected into the human nucleus pulposus cell to activate telomerase and construct immortalized human nucleus pulposus cell line through induction immortalized human nucleus pulposus cell line, normal cells not transformed cells are used, wherein the cells have normal growth rate and karyotype, have contact inhibition, anchorage dependence and other safety properties and do not have carcinogenicity and soft agar cloning capability; and as same as the germline stem cells, the cells have real immortalization, thus the standard cell line can be provided for the further research of the pathogenic mechanism of the degenerative disc disease and a new idea is provided to increase the entire control level of the degenerative disc disease.
Owner:梁伟国
Method for preparing jarosite through microbial conversion
InactiveCN103740763AEasy to manufactureQuick and easy to manufactureMicroorganism based processesFermentationBiotechnologyMicroorganism
The invention relates to a method for preparing jarosite through microbial conversion. The method comprises the following steps: (1) preparing a 9K culture medium; (2) preparing a microbial conversion culture medium; (3) preparing a soft agarose culture medium; (4) performing culture preservation, namely mixing leptospirillum ferriphilum BYQ bacteria liquid in a stable phase preserved at the temperature of 0 DEG C with the soft agarose culture medium, solidifying at room temperature, and preserving at the temperature of 2-4 DEG C; (5) activating the strain, namely inoculating the leptospirillum ferriphilum BYQ strain into a shake flask containing the 9K culture medium and performing shaking culture, thus obtaining a strain activation solution; (6) performing biological conversion, namely inoculating the strain activation solution to a shake flask containing the microbial conversion culture medium and performing shaking culture, and standing at room temperature, thus obtaining jarosite which is attached to the wall of the shake flask and is generated through biological conversion; (7) soaking and centrifuging the shake flask to which the jarosite is attached by using a hydrochloric acid solution, thus obtaining a precipitate; and (8) performing vacuum drying on the precipitate to constant weight, and grinding to obtain the jarosite. The method is rapid, simple, convenient and environmentally friendly.
Owner:LANZHOU UNIVERSITY
Anti-tumor effects of prostate carcinoma tumor antigen-1
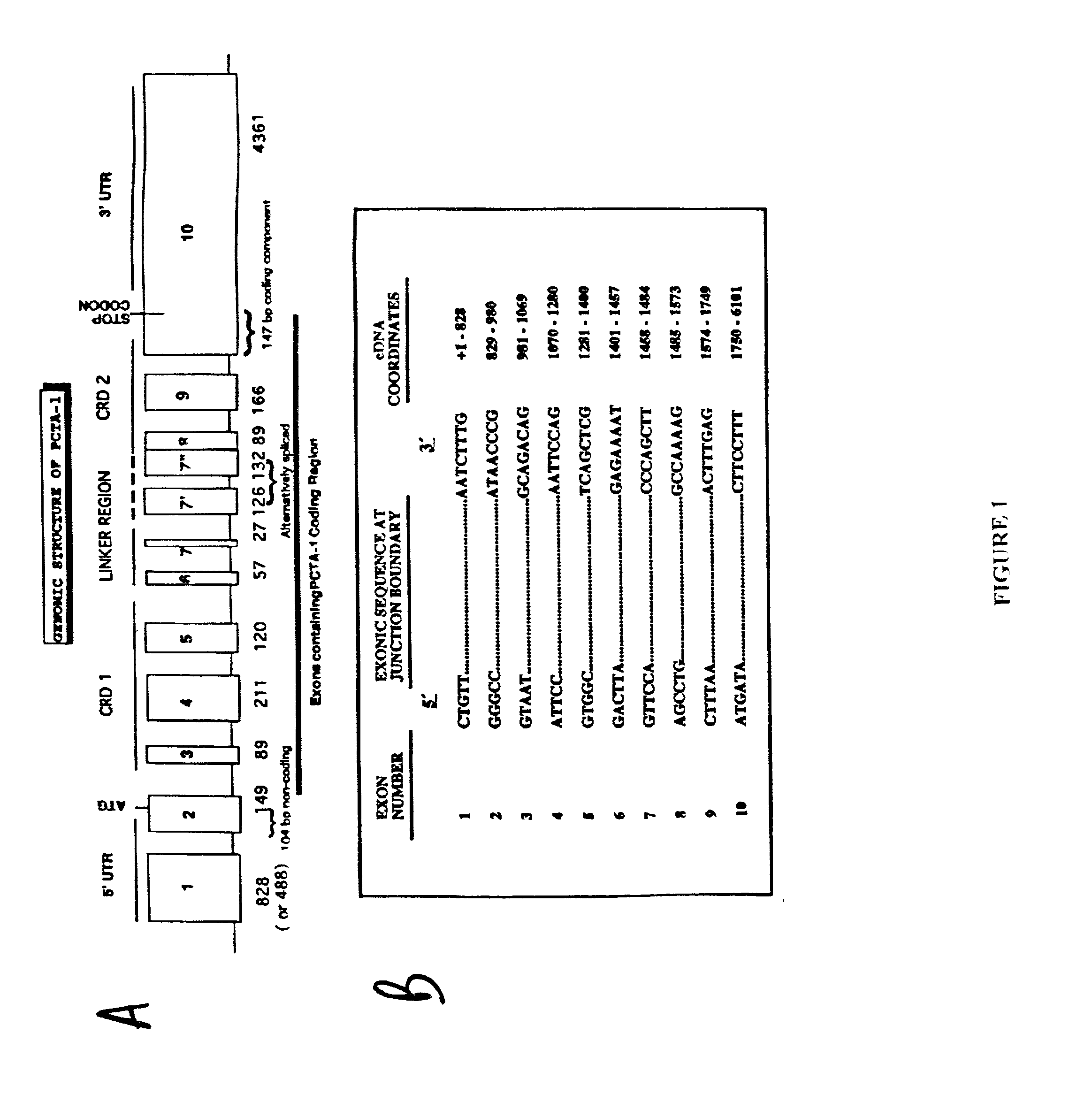
InactiveUS20030050266A1Prevent proliferationInhibiting metastasisVirusesPeptide/protein ingredientsAbnormal tissue growthLymphatic Spread
The present invention relates to methods of inhibiting the proliferation and / or metastasis of a cancer cell by administering, to the cancer cell, a molecule which increases, in the cell or at the cell surface, the amount of a Bivalent Prostate Carcinoma Tumor Antigen-1 ("B-PCTA-1") protein (referred to as "bivalent" because it comprises both carbohydrate recognition domains ("CRDs")). It is based, at least in part, on the discovery that increased expression of the full-length open reading frame of the PCTA-1 gene suppressed proliferation of tumor cells in soft agar (a characteristic associated with malignancy and tumor metastasis), whereas increased expression of a PCTA-1 gene lacking the second CRD-encoding region had the opposite effect, increasing the anchorage-independent proliferation of the tumor cells.
Owner:FISHER PAUL B +1
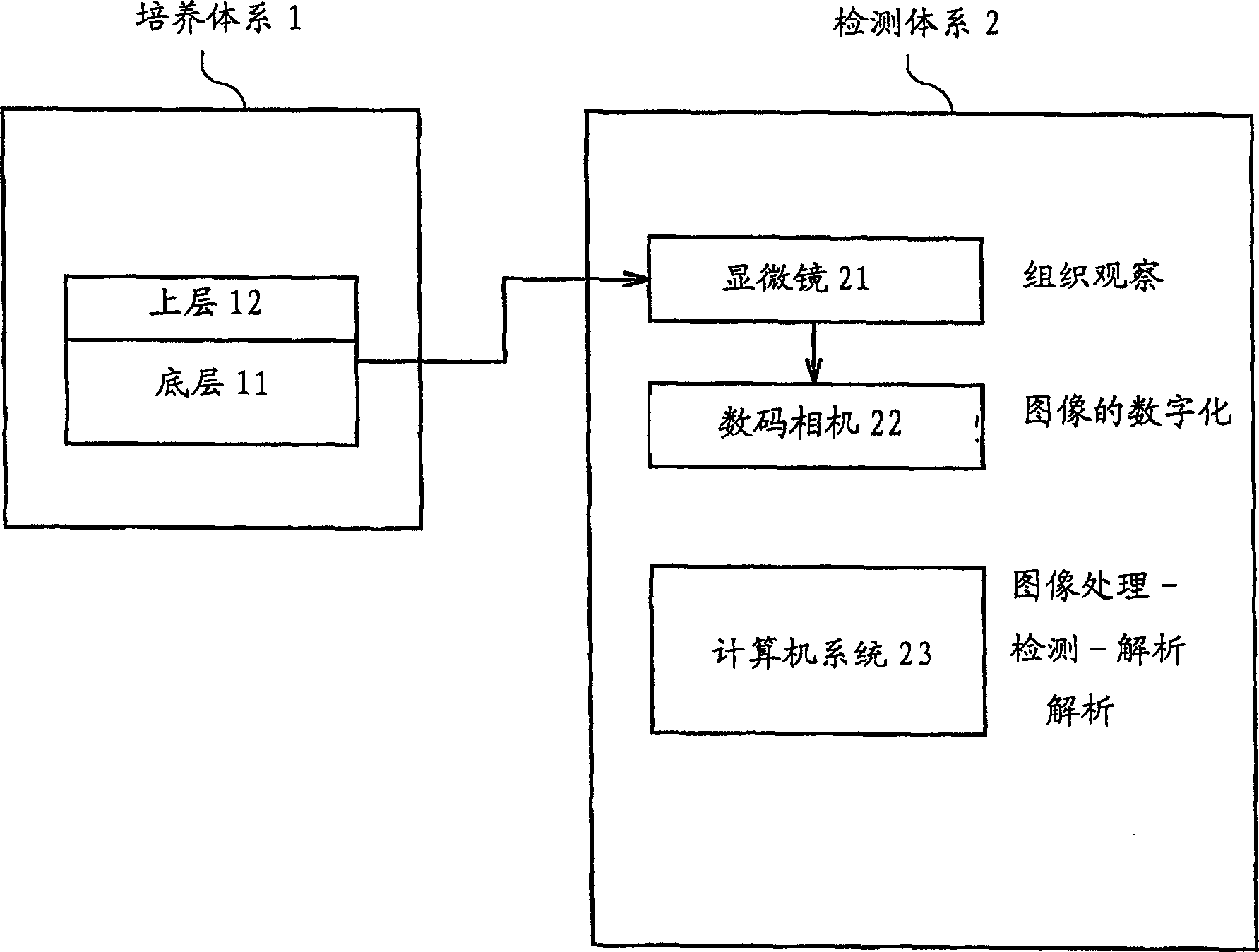
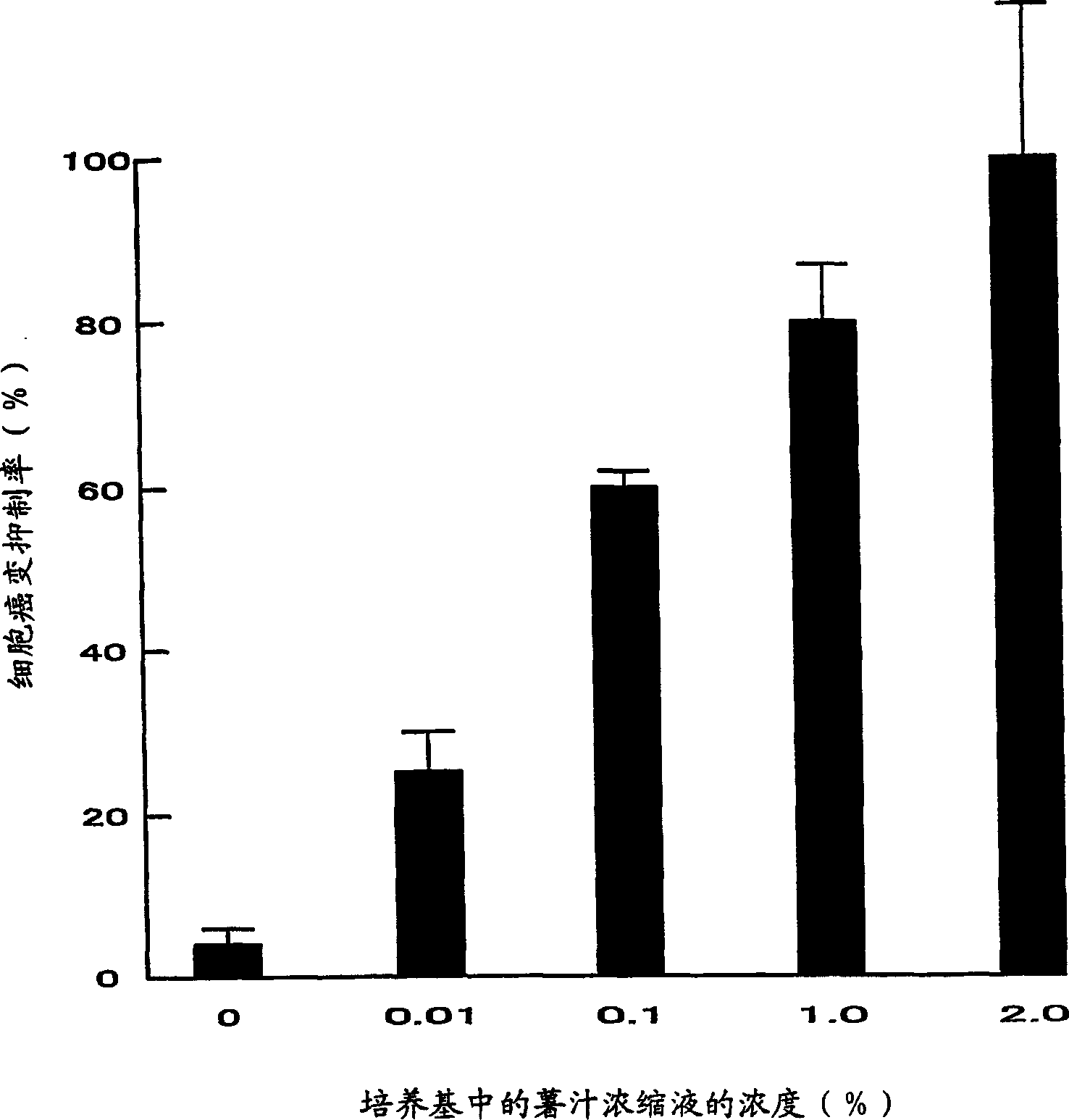
Culture system, detection and analysis system and detection method for cancer cell colonies
InactiveCN1678751ABioreactor/fermenter combinationsBiological substance pretreatmentsCarcinogenCancer cell
It is intended to provide a detection and analysis system for cancer cell colonies, whereby an environmental cell carcinogen causing cell carcinogenesis or a chemical or a food inhibiting cell carcinogenesis can be quickly and accurately detected, and a method therefor. Namely, a detection and analysis system for cancer cell colonies using a culture system (1) prepared by the agar overlaying method which comprises a bottom layer (1) being composed of a culture medium, soft agar and a carcinogenesis inducer and / or an anticancer agent and having a definite size and a top layer (12) being composed of a culture medium, soft agar and cells, and further provided with an optical microscope (21), an electronic data conversion unit (22) such as a digital camera and a computer system (23) for processing the data converted by the electronic data conversion unit (22). This computer system (23) has an image analysis software stored therein whereby the electronic data are grayed, calibrated and binarized by subtraction and single threshold and thus the presence or absence of colonies, the number of colonies, the size distribution of colonies, etc. are analyzed.
Owner:侯德兴 +2
Two breast cancer stem cell lines capable of being stably cultured and keeping original characteristics, separation method and uses thereof
InactiveCN105274059AStable and repeatableSafe and effective killGenetic material ingredientsTumor/cancer cellsAbnormal tissue growthPenicillin
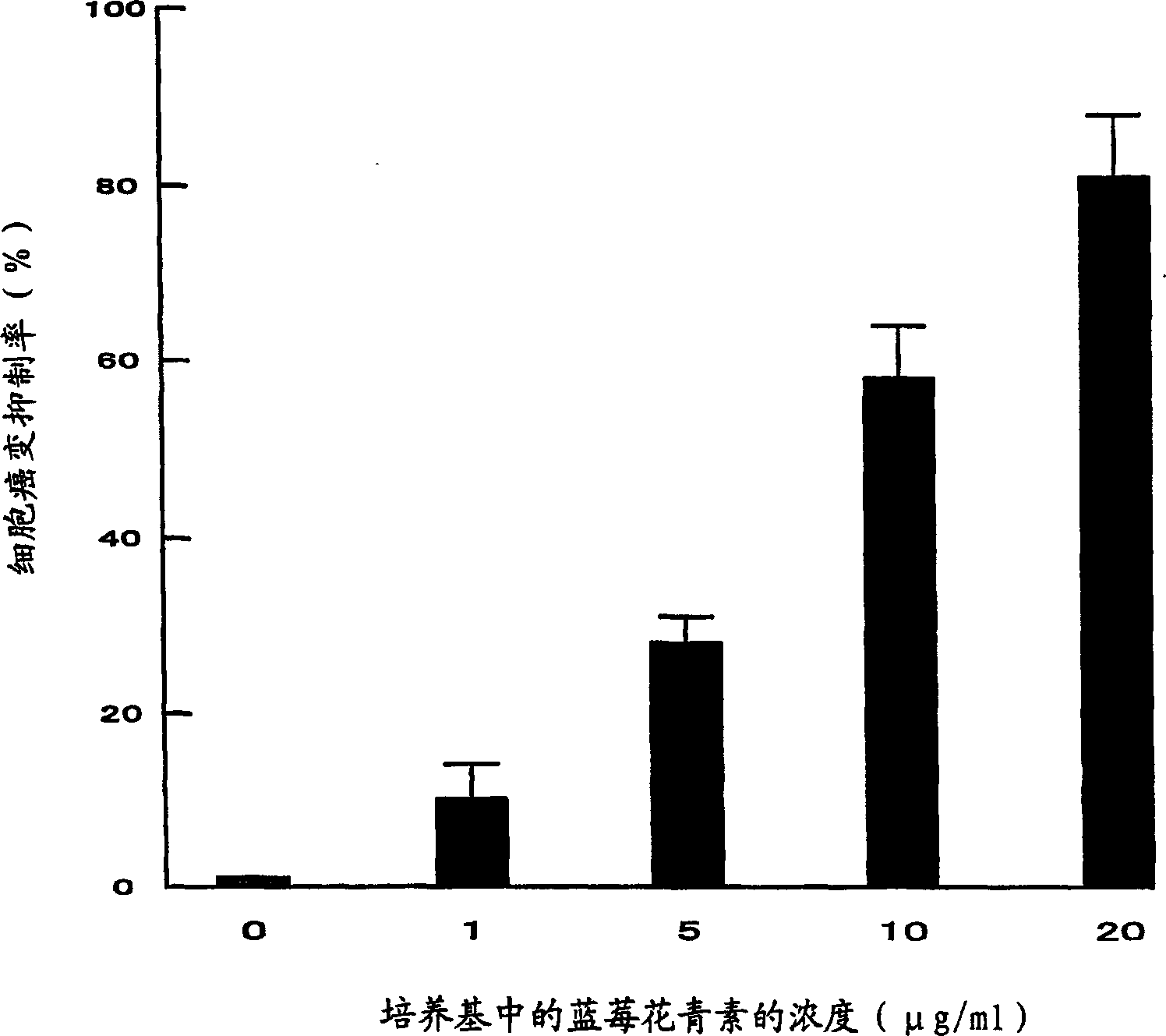
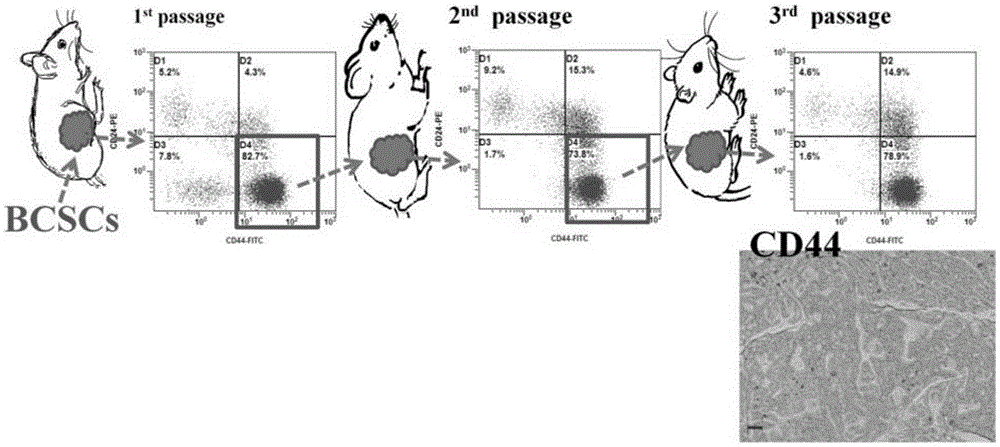
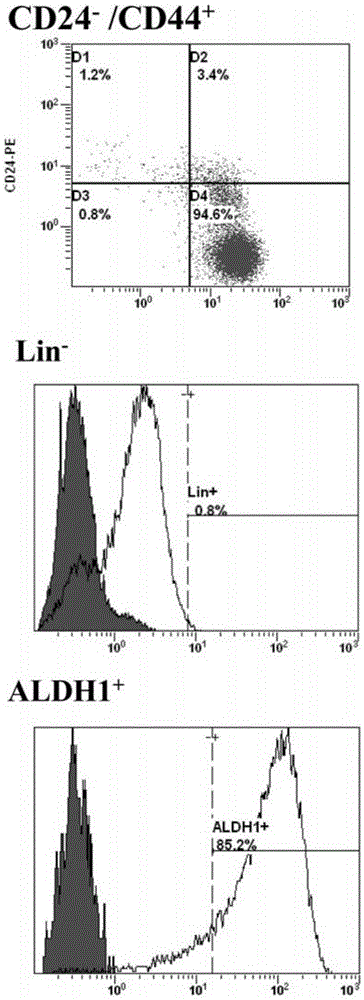
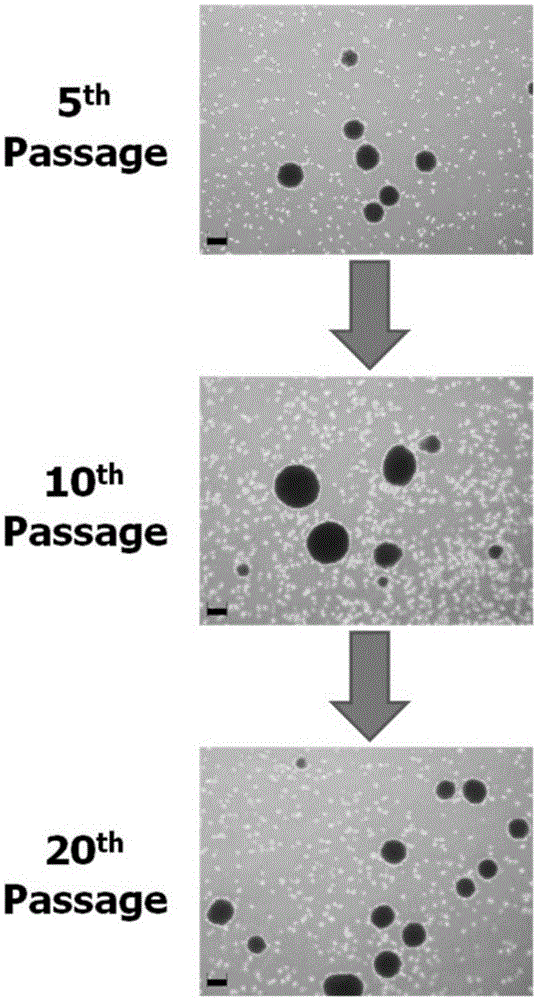
The invention discloses two breast cancer stem cell lines capable of being stably cultured and keeping original characteristics, separation method and uses thereof. A breast cancer tumor specimen just cut off from the tumor is taken, then is subjected to collagenase digestion and is filtered; a flow cytometer sorts CD44<+>, CD24<-> and ALDH1<+> cells, the cells are adjusted to a purity of more than 98%; a stem cell culturing agent for breast cancer cell is prepared; the sorted cells are screened in a monoclonal manner and the obtained monoclone cells are inoculated in an ultralow adhesion culture dish containing breast cancer stem cell culturing agents; penicillin and streptomycin are added in the original culturing medium; culturing is performed in CO<2> cultivation box. In the continuous culturing process, the breast cancer stem cell lines capable of being stably cultured and keeping original characteristics keep ball formation ability, self-updating ability, CD44<+>, CD24<-> / low Lin- ALDH1<+> phenotype, pluripotency differentiation ability, in vitro soft agar tumorigenesis ability, in vivo tumorigenesis ability in nude mouse, and in vivo tumorigenesis pluripotency differentiation ability in nude mouse.
Owner:SUN YAT SEN UNIV CANCER CENT
Nucleophosmin/B23-binding peptide to inhibit tumor growth and regulate transcriptional activity of p53
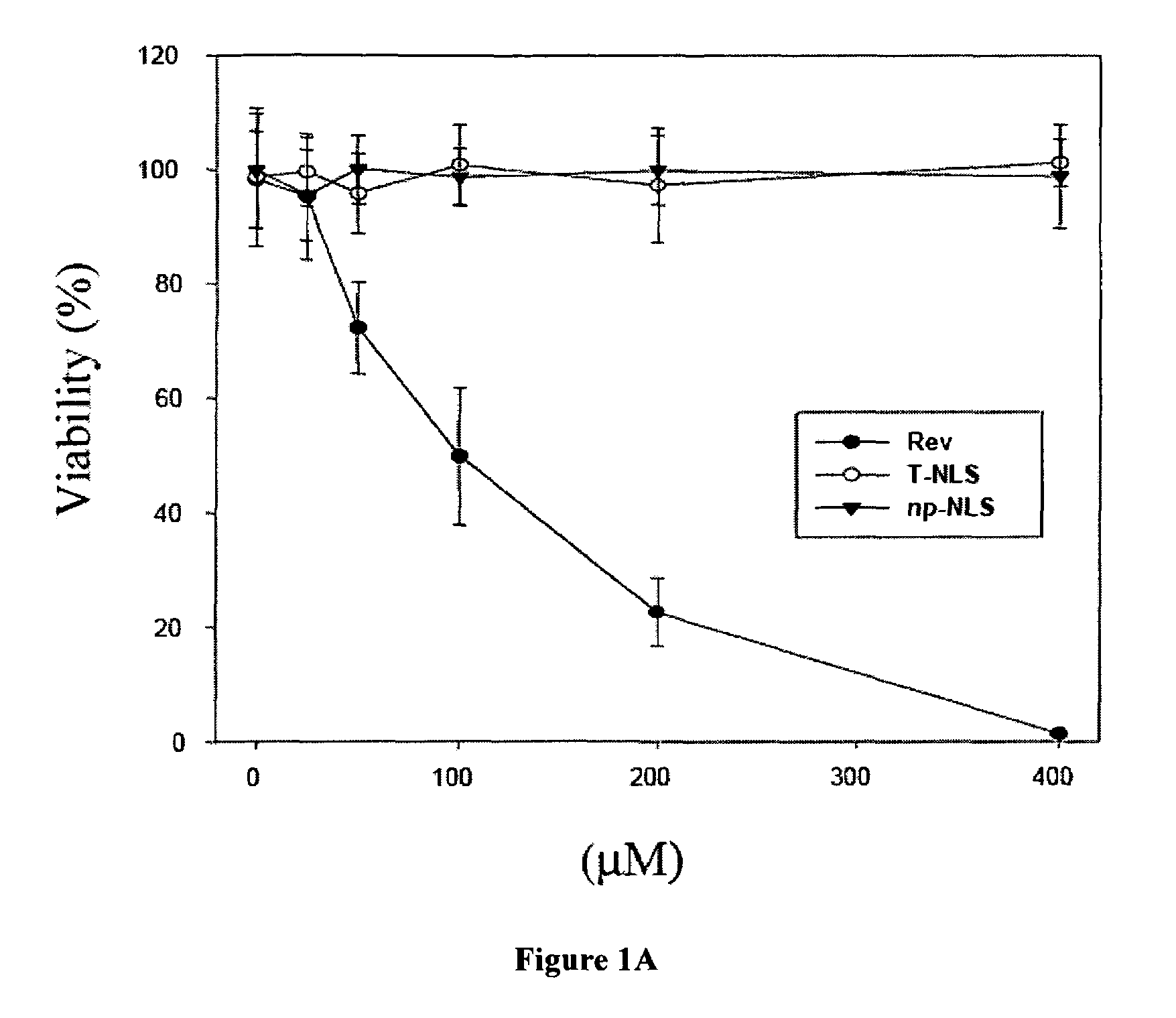
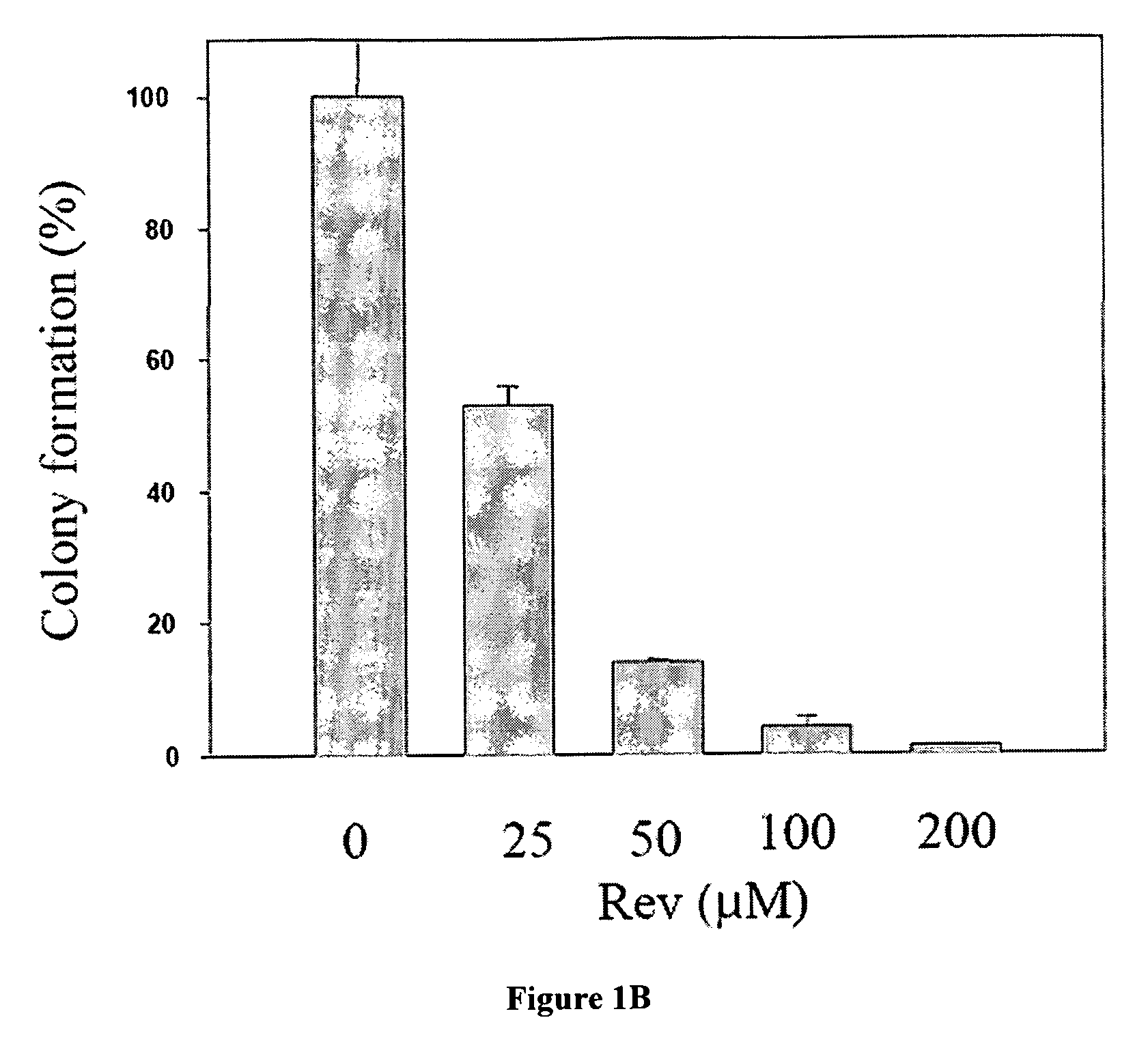
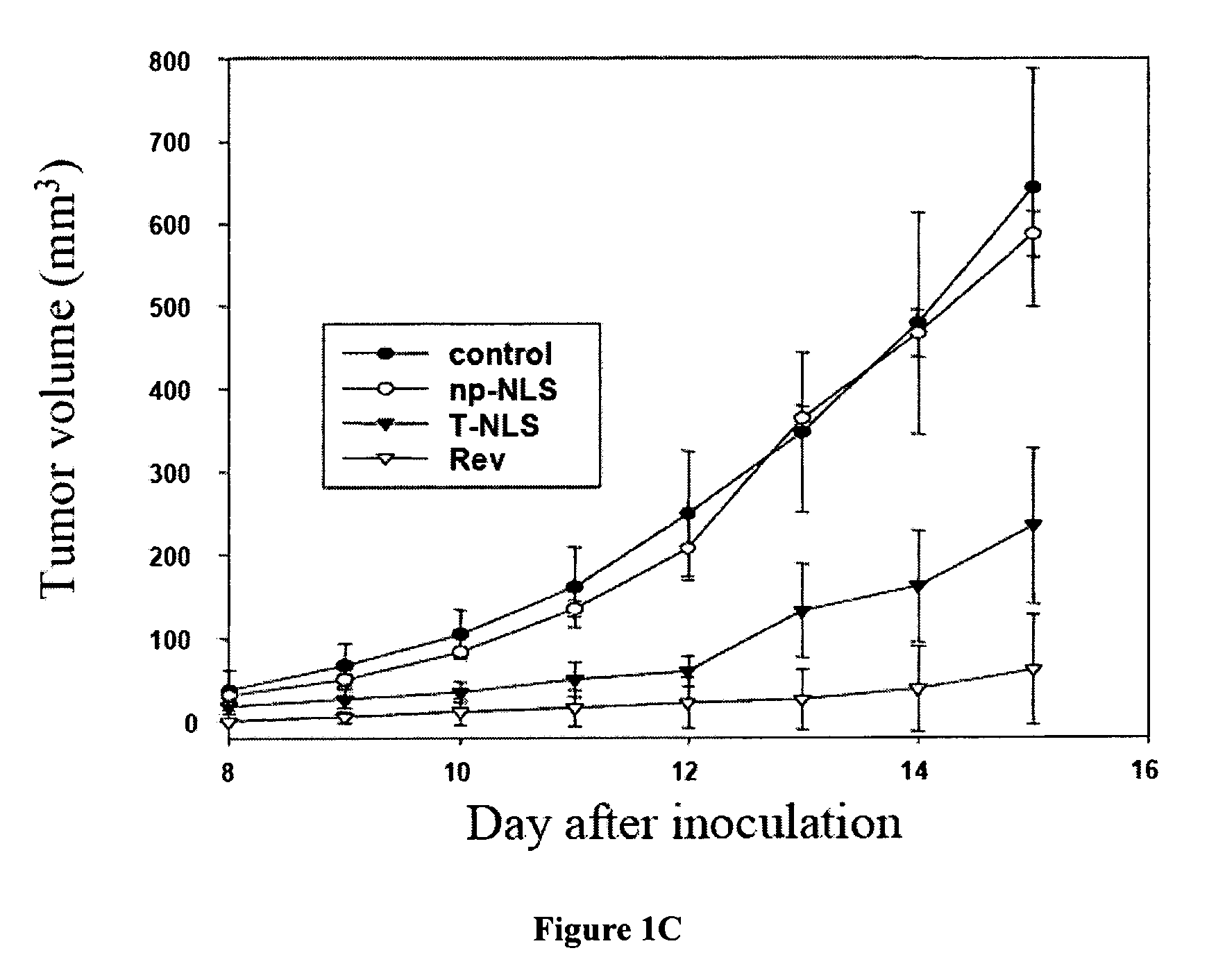
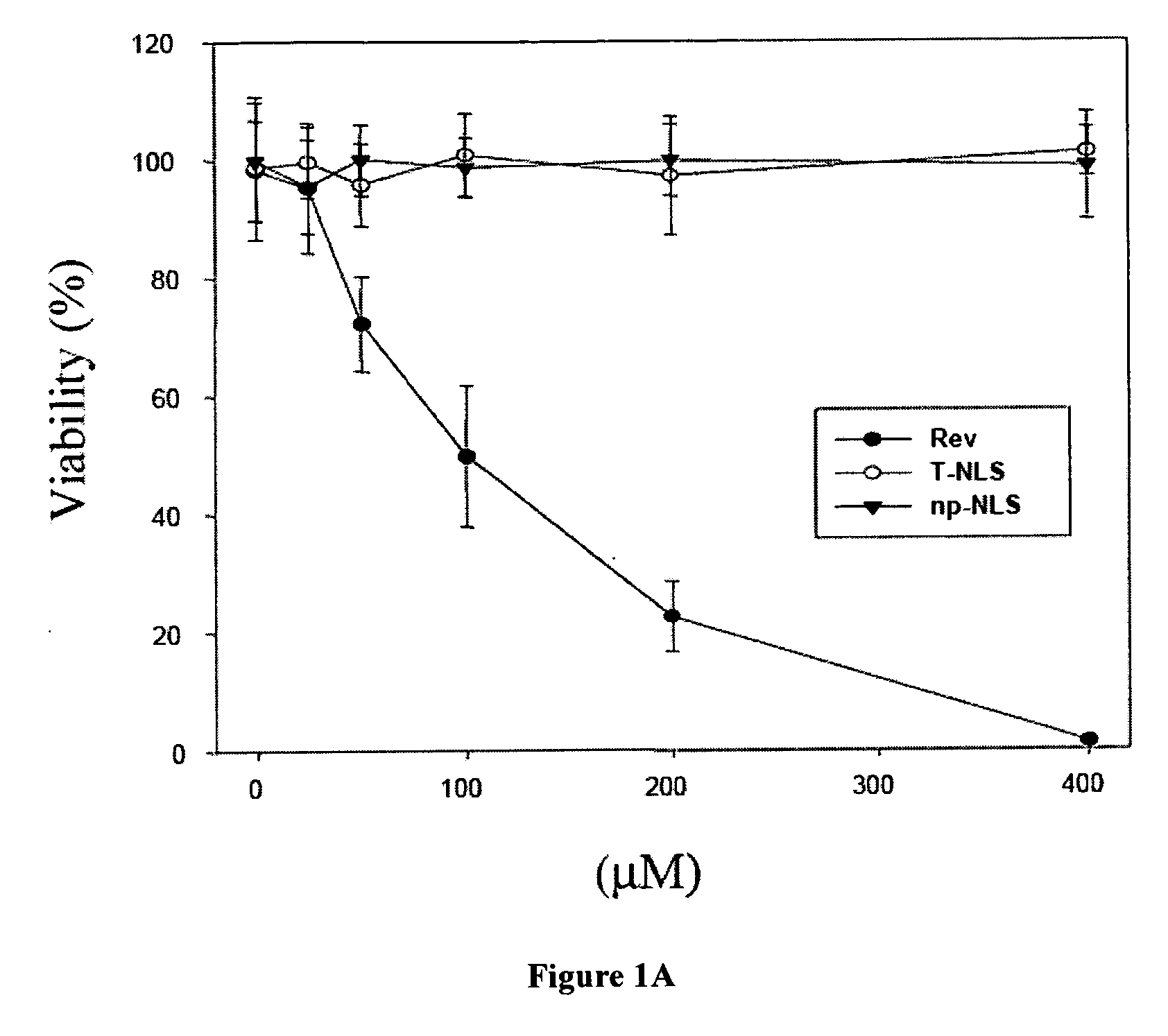
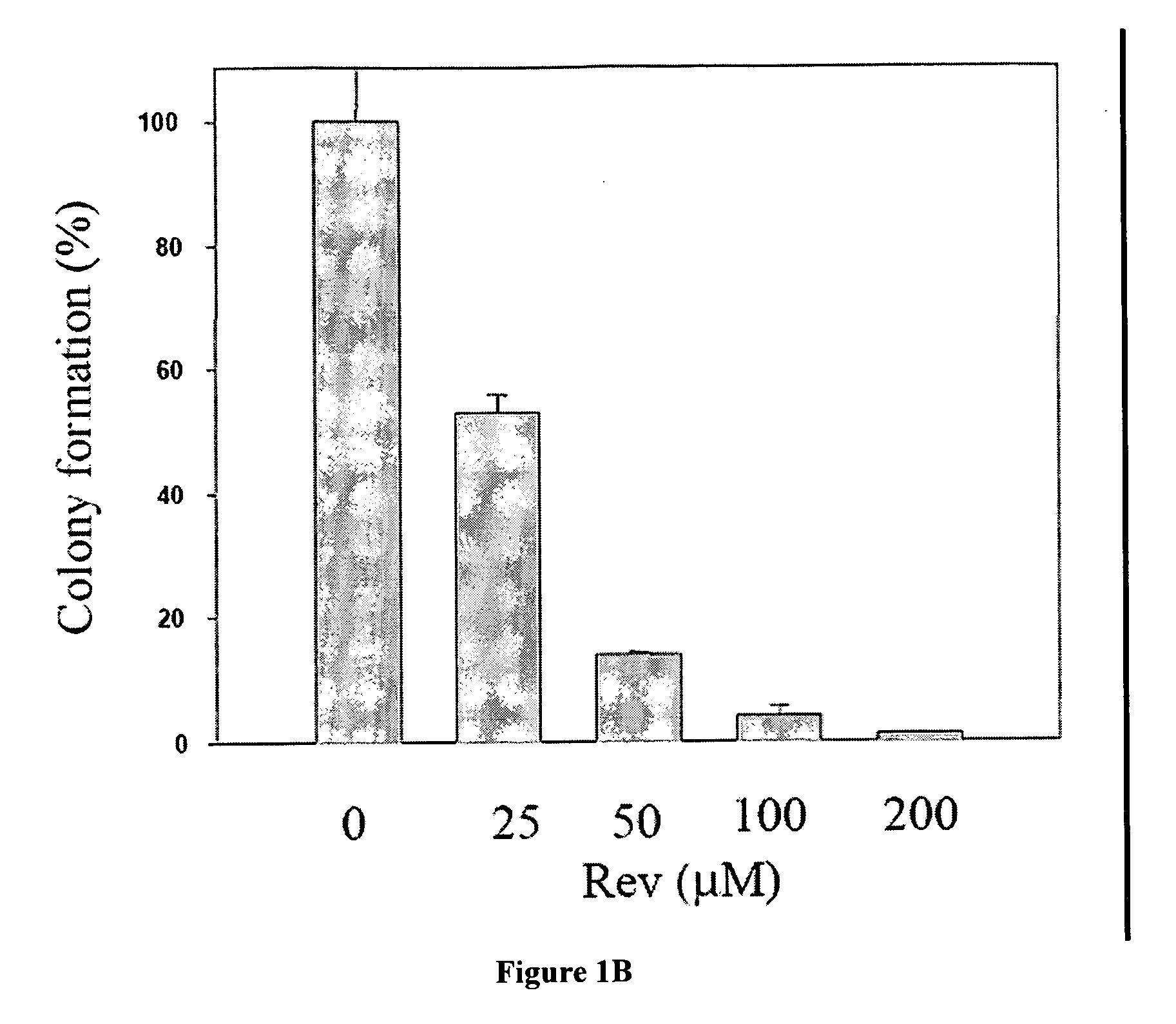
The Rev peptide that binds to nucleophosmin / B23 with the highest affinity exhibits the greatest cytotoxicity on Ras-3T3 cells and inhibits tumor growth most effectively in nude mice. The efficiency of colony formation in soft agar of Ras-3T3 cells is significantly inhibited by treatment with Rev peptide. In addition, Rev peptide can potentiate the doxorubicin-induced decrease of cellular viability in U1 bladder cancer cells and inhibition of tumor growth in nude mice. Treatment of Rev peptide increases protein expression and transcriptional activity of p53 and inhibits the nucleophosmin / B23-mediated PCNA promoter activation. Peptides having high affinity of binding to molecular targets such as nucleophosmin / B23 represent a useful approach to anti-cancer biotherapeutics.
Owner:CHANG GUNG UNIVERSITY
Screening method for lactic acid bacteria
PendingCN109837323AHigh screening accuracyStrong targetingMicrobiological testing/measurementMicroorganismLactobacillus
The invention relates to the technical field of microbial screening, and in particular, relates to a screening method for lactic acid bacteria. The method includes the steps: grinding a sample firstly, and then carrying out enlarged cultivation; then growing on a culture medium by a tilt-pour process so as to separate single colonies of lactic acid bacteria; inoculating an MRS flat plate with indicator bacteria with single colonies by a bacteria dotting manner, and covering with MRS soft agar; selecting strains with a bacteriostatic effect, and carrying out purification and preservation of thesingle colonies; and finally, verifying. The strains separated and screened by the method not only can reduce the impact of other miscellaneous bacteria in early stage, but also can eliminate the non-bacteriostatic lactic acid bacteria, can reduce the workload, also can screen the required strains for inhibiting pathogenic bacteria more pertinently.
Owner:GUANGZHOU INST OF ADVANCED TECH CHINESE ACAD OF SCI +2
Nucleophosmin/B23-binding peptide to inhibit tumor growth and regulate transcriptional activity of p53
InactiveUS20070254845A1High binding affinityInhibit tumor growthAntibacterial agentsBiocideCytotoxicityBinding peptide
The Rev peptide that binds to nucleophosmin / B23 with the highest affinity exhibits the greatest cytotoxicity on Ras-3T3 cells and inhibits tumor growth most effectively in nude mice. The efficiency of colony formation in soft agar of Ras-3T3 cells is significantly inhibited by treatment with Rev peptide. In addition, Rev peptide can potentiate the doxorubicin-induced decrease of cellular viability in U1 bladder cancer cells and inhibition of tumor growth in nude mice. Treatment of Rev peptide increases protein expression and transcriptional activity of p53 and inhibits the nucleophosmin / B23-mediated PCNA promoter activation. Peptides having high affinity of binding to molecular targets such as nucleophosmin / B23 represent a useful approach to anti-cancer biotherapeutics.
Owner:CHANG GUNG UNIVERSITY
Construction of 18-trisomy syndrome cell model and cell bank of 18-trisomy syndrome cell by SV40LT gene transfection
InactiveCN104419684AReduce injuriesIncrease success rateMicroorganism librariesVector-based foreign material introductionEscherichia coliTrisomy X syndrome
The invention relates to construction of an 18-trisomy syndrome cell model and a cell bank of 18-trisomy syndrome cell by SV40LT gene transfection for the field of medical research. The construction is mainly characterized in that an SV40LTag-pcDNA3.1(-) recombinant is constructed through a T4DNA ligase, BamHI, pcDNA3.1(-) DNA and SV40LTag DNA as a matter of routine, the recombinant is purified through competent escherichia coli, the recombinant is introduced into an in-vitro passage cell digested through collagenase II or an 18-trisomy syndrome tissue cell which is logarithmically grown through a lipoid transfection method to be integrated with cell DNA, and a cloned cell which is screened through G418 and contains a positive recombinant is subjected to amplification culture; and a cell which accords with the characteristics of an immortalized cell and is the same as or similar to a primary cell on a cell morphology, a growth curve, a chromosome karyotype, soft agar colony growth, a nude mouse tumorigenesis test, SV40 large T gene detection of transfection cell DNA, an mRNA expression product measurement result and a DNA sequence measurement result is screened as the 18-trisomy syndrome in-vitro research cell model and cryopreserved in liquid nitrogen, so that the foundation is laid for researching the pathogenesis of 18-trisomy syndrome for a long time from cell level in vitro.
Owner:翁炳焕
Quantitative soft agar colony formation assay using tetrazolium that generates water-soluble formazan
InactiveUS20140248655A1Improve accuracyImprove efficiencyCompound screeningApoptosis detectionFormazanTetrazole
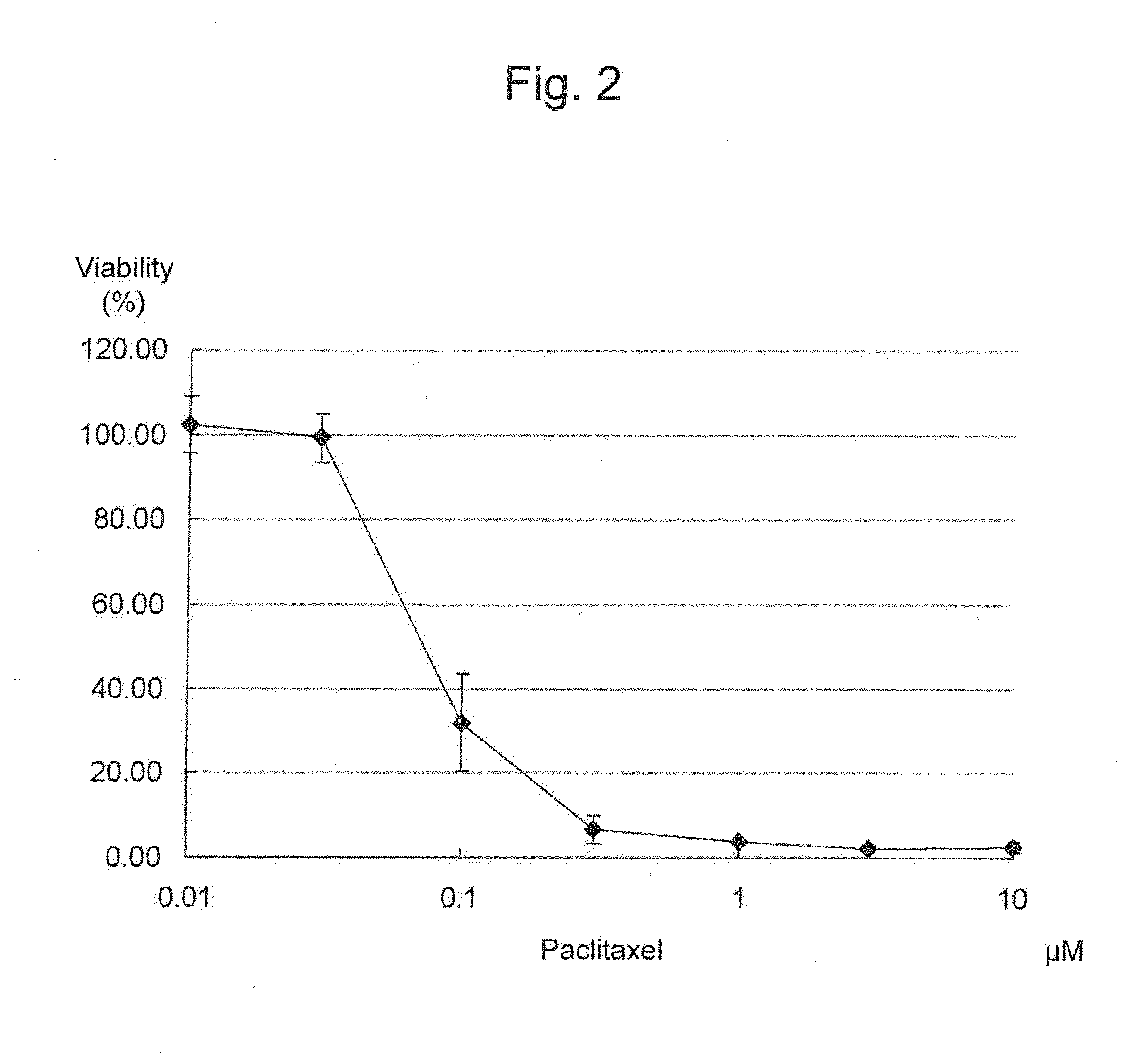
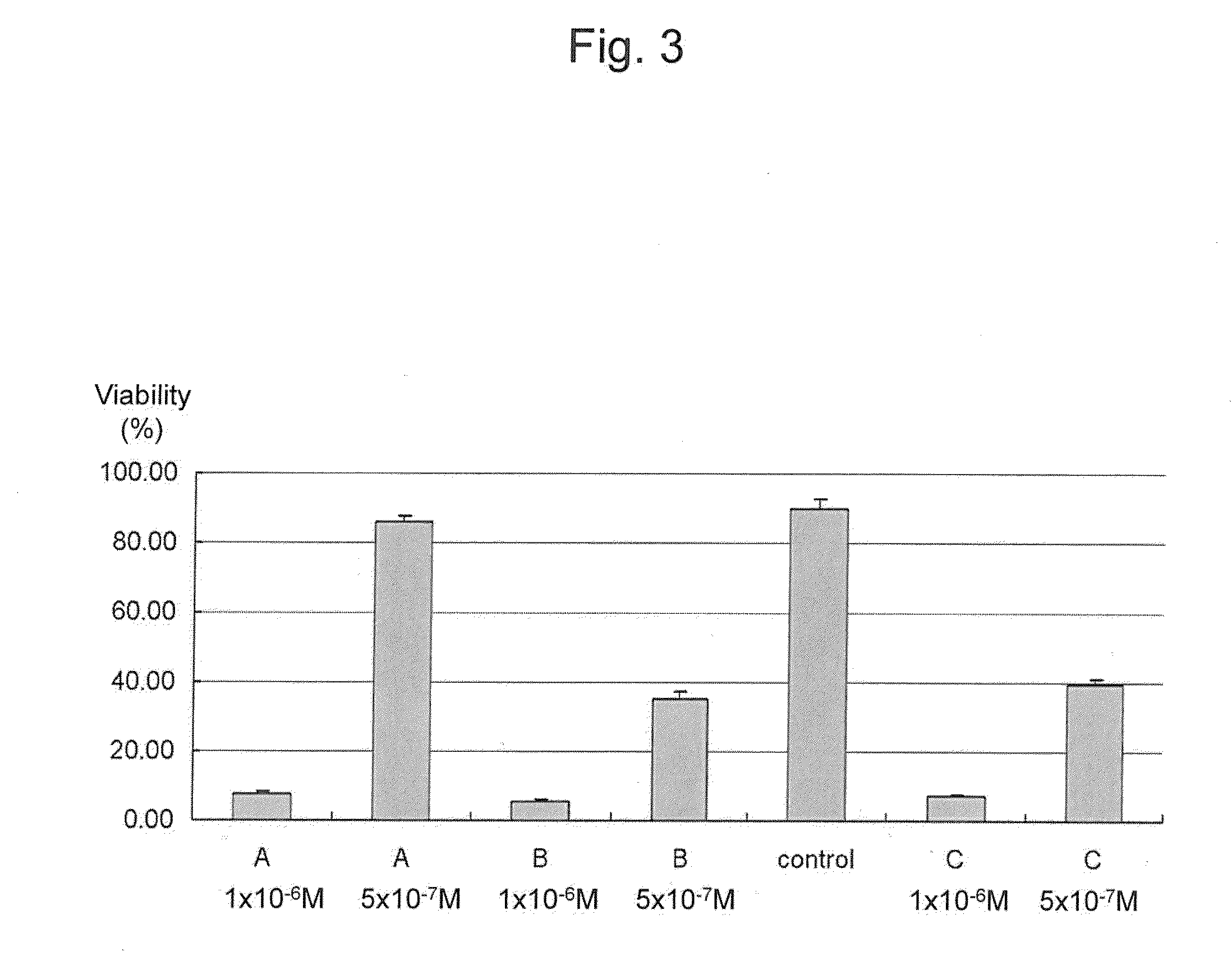
This invention provides a quantitative assay technique for soft agar colony formation that can be carried out rapidly with high accuracy. Specifically, this invention relates to a method for evaluation of cell survival comprising: a step of overlaying agar at the bottom of a vessel with agar containing cells, overlaying the agar containing cells with a medium and culturing the cells; a step of removing the medium, adding tetrazolium that produces water-soluble formazan and an electron carrier and culturing the cells; and a step of evaluating cell survival based on the color developed by the water-soluble formazan.
Owner:KAGOSHIMA UNIV +1
Kit for salmonella paratyphi B and salmonella paratyphi B var Java, as well as preparation and using method of kit
ActiveCN105004857ARapid Identification EffectSimple identification effectBiological material analysisBiotechnologySodium potassium tartrate
The invention relates to an identification method for salmonella paratyphi B and salmonella paratyphi B var Java in salmonella species, and mainly solves the technical problems of rapid screening and identification of salmonella paratyphi B from clinical diarrhea cases and environment and food samples. According to the technical scheme, a kit for identifying salmonella paratyphi B and salmonella paratyphi B var Java is adopted. The kit is characterized by comprising the following raw materials: (1) a salmonella B group immune rabbit anti-serum which contains diluted serums of salmonella O:4 and O:5 factor antibodies; (2) a salmonella H-phase immune rabbit anti-serum which contains diluted serums of salmonella H:b and H:2 factor antibodies; (3) a potassium tartrate biochemical identification tube which contains 10.0 g of peptone, 10.0 g of sodium potassium tartrate, 5.0 g of sodium chloride, 0.024 g of phenol red, 15.0 g of agar and 1000.0 ml of distilled water; (4) sterilizing mineral oil; (5) H-phase dedicated inducing soft agar which contains 10.0 g of tryptic soy agar, 5.0 g of casein, 1.0 g of potassium nitrate, 2.0 g of agar powder and 1000.0 ml of distilled water; (6) an H:b factor inducing serum which contains inducing serum H:b factors.
Owner:上海市长宁区疾病预防控制中心 +1
Establishment method of tonalid-induced human normal hepatocyte malignant transformation model
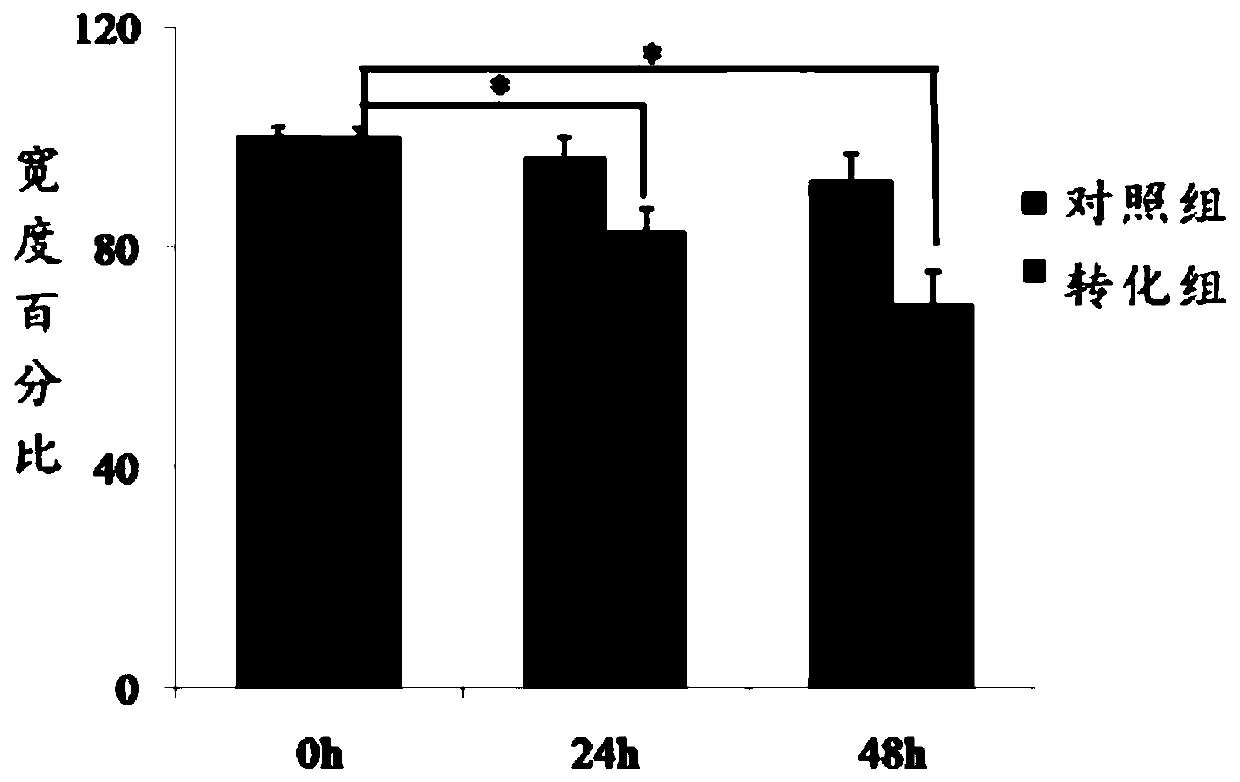
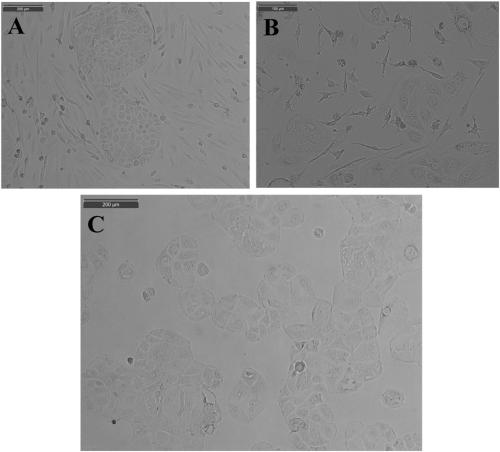
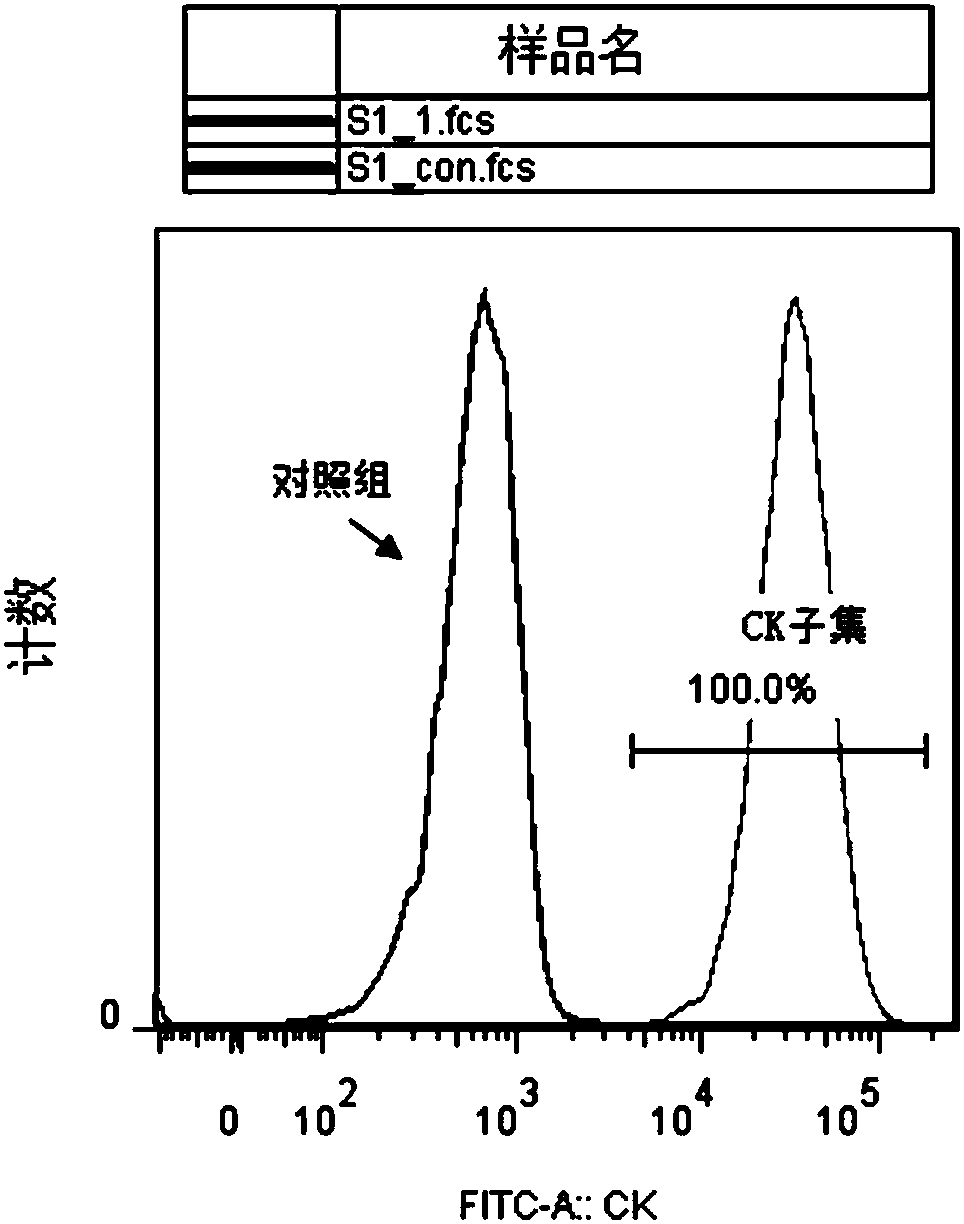
InactiveCN110699323AScratch heals fastImprove migration abilityArtificial cell constructsCell culture active agentsClone (cell biology)Human body
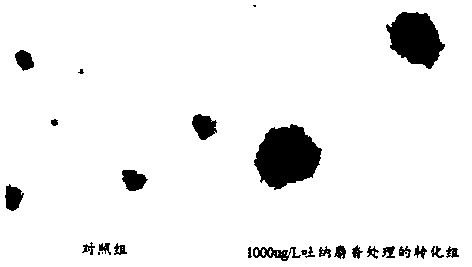
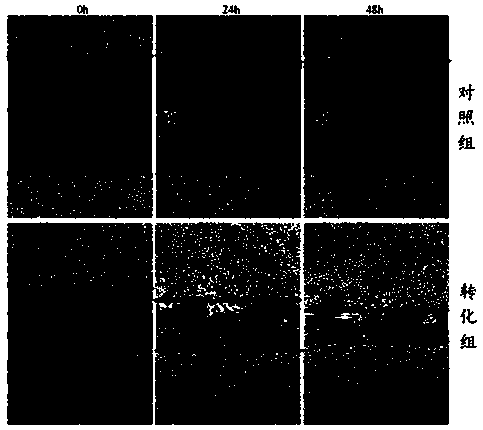
The invention belongs to the field of cell biology, and provides an establishment method of a tonalid-induced human normal hepatocyte malignant transformation model. The establishment method comprisesthe steps: a tonalid working solution is prepared; L02 human body normal hepatocyte is cultured to the logarithmic phase and then divided into a transformation group and a control group; and cells inthe transformation group are treated through tonalid; the treated cells in the transformation group and the treated cells in the control group are cultured to 20 generations, the anchoring independent growth capacity of the cells is detected through a soft agarose cloning experiment, and then the healing and migration capacity of the cells is detected through a scratch experiment. According to the establishment method provided by the invention, clone cell clusters with the large size and the orderly edge can be observed in the transformation group through the soft agarose cloning experiment,through the scratch experiment, it can be observed that scratches of the clone cells in the transformation group is healed more rapidly than scratches of the cells in the control group, the cell migration capacity is higher, it is effectively verified that the tonalid has a carcinogenic effect on human bodies, and the reference basis is further provided for research of the toxic effect of the tonalid; and the method is easy to operate, convenient and low in cost.
Owner:XIAMEN UNIV +1
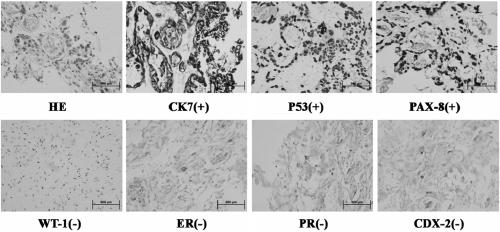
Human cervical adenocarcinoma cell line and preparation method and application thereof
ActiveCN109929804AProliferate fastHigh selectivityMicrobiological testing/measurementMicroorganism based processesCancer cellResistant strain
The present invention provides a human cervical adenocarcinoma cell line, which is characterized by naming as a primary cervical adenocarcinoma drug-resistant strain W038, and is deposited in the China Center for Type Culture Collection with a preservation number being CCTCC No: C2017246. The primary cervical adenocarcinoma drug-resistant strain W038 has weak invasion ability and weak migration ability, and can be used as a double-negative control group for invasion and migration. The in-vitro soft agar colony formation test proves that the tumorigenicity proves that the cloning ability is strong, and the in-vitro susceptibility test that the strain is resistant to a variety of drugs, which is consistent with clinical features. The preparation method disclosed by the invention can purify cancer cell lines to over 99%, wherein the screened medium component can selectively kill histocyte, has high selectivity for tumor cell growth, and can increase the proliferation rate of tumor cells and shorten the culture period. The method can be used not only to purify the cervical adenocarcinoma lines, but also to purify and prepare other cancer cell lines.
Owner:TIANJIN MEDICAL UNIV CANCER INST & HOSPITAL
Targeting metabolic enzymes in human cancer
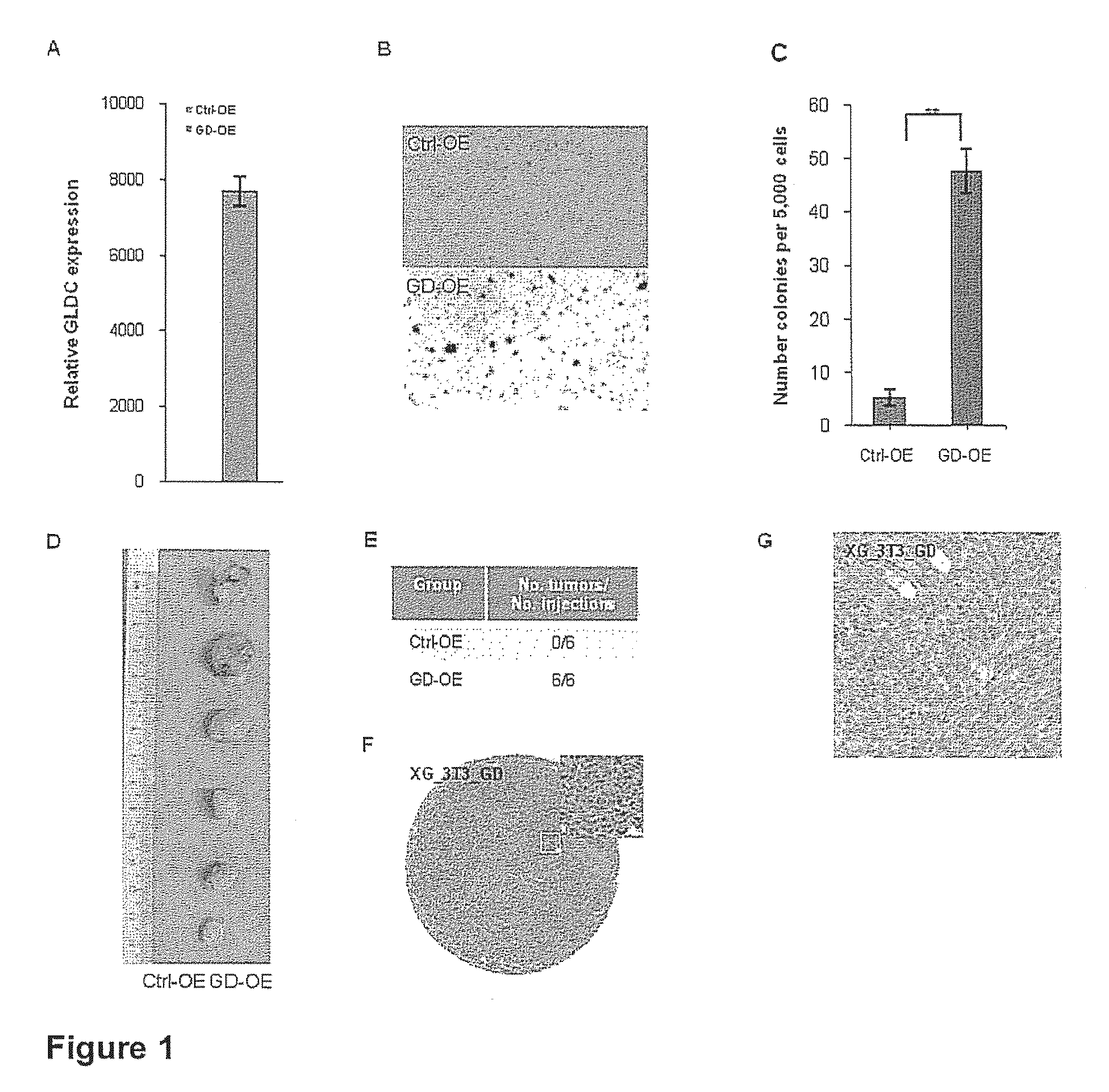
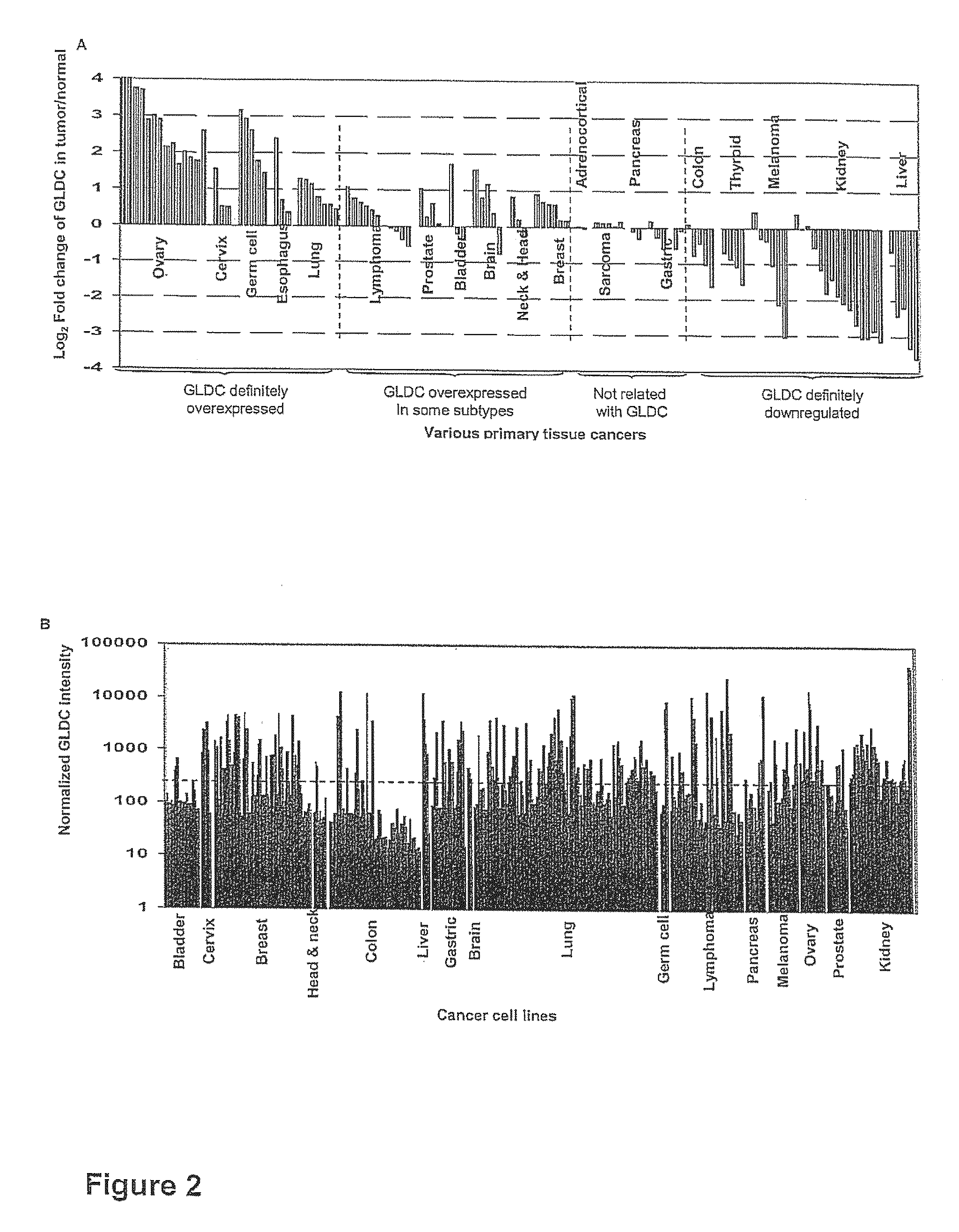
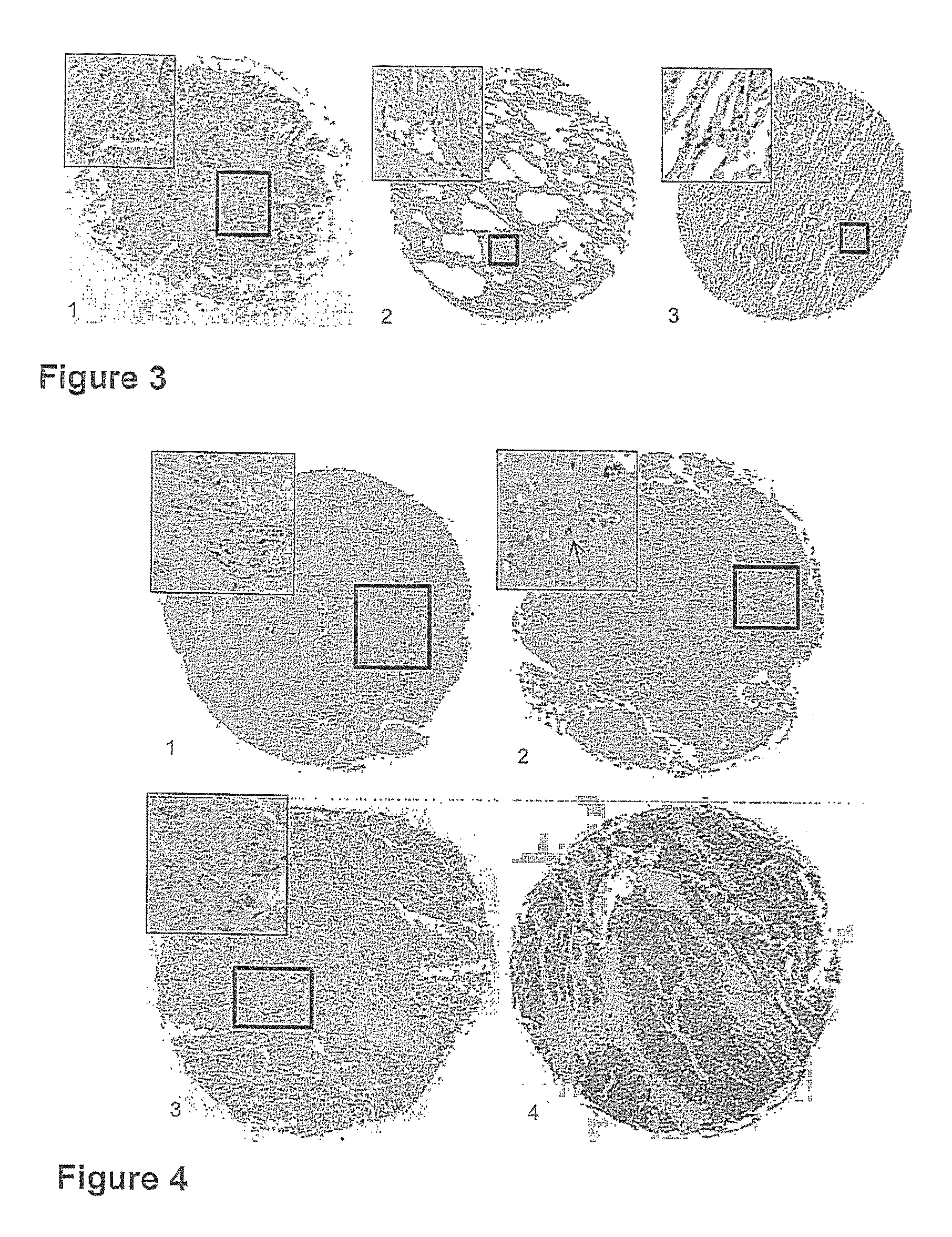
Targeting metabolic enzymes in human cancer Abstract Lung cancer is a devastating disease and a major therapeutic burden with poor prognosis. The functional heterogeneity of lung cancer (different tumor formation ability in bulk of tumor) is highly related with clinical chemoresistance and relapse. Here we find that, glycine dehydrogenase (GLDC), one of the metabolic enzyme involved in glycine metabolism, is overexpressed in various subtypes of human lung cancer and possibly several other types of cancers. GLDC was found to be highly expressed in tumor-initiating subpopulation of human lung cancer cells compared with non-tumorigenic subpopulation. By array studies we showed that normal lung cells express low levels of GLDC compared to xenograft and primary tumor. Functional studies showed that RNAi inhibition of GLDC inhibits significantly the clonal growth of tumor-initiating cells in vitro and tumor formation in immunodeficient mice. Overexpression of GLDC in non-tumorigenic subpopulation convert the cells to become tumorigenic. Furthermore, over-expression of GLDC in NIH / 3T3 cells and human primary lung fibroblasts can transform these cells, displaying anchorage-independent growth in soft agar and tumor-forming in mice. Not only is GLDC is expressed human lung cancer, it is also up-regulated in other types of cancer, such as colon cancer. RNAi knockdown of GLDC in colon cancer cell line, CACO-2 cells, can also inhibit the tumor formation in mice. Thus GLDC maybe a new metabolic target for treatment of lung cancer, and other cancers.
Owner:AGENCY FOR SCI TECH & RES
Building of klinefelter syndrome cell model and cell bank of klinefelter syndrome cell by virtue of recombinant SV40 gene
InactiveCN104419669AReduce injuriesIncrease success rateMicroorganism librariesVector-based foreign material introductionEscherichia coliPrimary cell
The invention relates to building of an SV40LT antigen gene-mediated klinefelter syndrome cell model and a cell bank of the klinefelter syndrome cell model, applied to the field of medical research. The building method is mainly characterized by comprising the following processes: building an SV40LTag-pcDNA3.1(1) recombinant by using T4DNA ligase, BamHI, pcDNA3.1(1) DNA and SV40LTag DNA according to a conventional method; purifying the recombinant by using competent escherichia coli, introducing the recombinant into an in vitro passage cell which is digested by collagenase II, or klinefelter syndrome histocyte which is in logarithmic growth, and integrating the recombinant with DNA; carrying out enlarging cultivation, screening cells containing positive recombinants by G418, and cloning; and screening a cell of which the cell morphology, the growth curve, karyotype, soft agar colony growth, nude mice tumor experiment result and transfection cell SV40 big T gene detection result, mRNA product measuring result and DNA sequencing result are in fit with the characteristics of an immortalized cell and are the same as or similar to that of a primary cell to be taken as an in vitro research cell model for the SV40LT-mediated klinefelter syndrome; and a foundation is laid for in vitro long-term research of pathogenesis of the klinefelter syndrome from the cellular level.
Owner:翁炳焕
Monoclonal antibody resisting ovarian cancer and application thereof
ActiveCN105462935AAbundant productionHigh potencyImmunoglobulins against animals/humansAntibody ingredientsAntigenNude mouse
The invention discloses a monoclonal antibody resisting the ovarian cancer and application thereof. A hybridoma cell strain NM003-1 secreting the monoclonal antibody resisting the human ovarian cancer is preserved in China Center for Type Culture Collection on September 26, 2011 with the preservation number of CCTCC NO: C201174. The hybridoma cell strain NM003-1 secretes the monoclonal antibody NJ003-1 resisting the human ovarian cancer. Expression of a specific antigen of the monoclonal antibody NJ003-1 in ovarian cancer tissue is higher than that in ovarian benign disease tissue; the positive expression rate of the antigen in a poorly differentiated patient is higher than that in a well differentiated patient. The monoclonal antibody NJ003-1 can effectively inhibit clone formation of ovarian carcinoma cells SK-OV-3 in soft agar and can remarkably inhibit growth of human ovarian cancer transplantation tumors in a nude mouse. Obviously, the monoclonal antibody is expected to become medicine for treating the ovarian cancer or to be prepared into a diagnostic reagent for detecting the ovarian cancer.
Owner:潘世扬
Method for rapidly screening space mutation variant strains of myxobacteria
PendingCN114134082ARealize screeningImprove screening efficiencyBacteriaMicroorganism based processesBiotechnologyMyxobacteria
The invention discloses a method for rapidly screening space mutation variant strains of myxobacteria. The method comprises the following steps: inoculating myxobacteria subjected to space mutation to a soft agar culture medium for screening for culture, and limiting the motion behavior of the myxobacteria through soft agar, so as to screen the space mutation variant strains of the myxobacteria. Tests show that the myxococcus flavus A motion-deficient mutant is obtained by adopting the method disclosed by the invention; adding milk as an indicator to obtain a variant strain with improved milk degradation capability; a bacterial strain with bacterial colony color variation is screened by adding Congo red. By adopting the method disclosed by the invention, the screening of variant strains with excellent performance can be quickly realized, and the screening efficiency of the myxococcus flavus variant strains is improved.
Owner:GUANGDONG INST OF MICROBIOLOGY GUANGDONG DETECTION CENT OF MICROBIOLOGY
Application of SETDB1 to adjusting and controlling of cholesterol ways
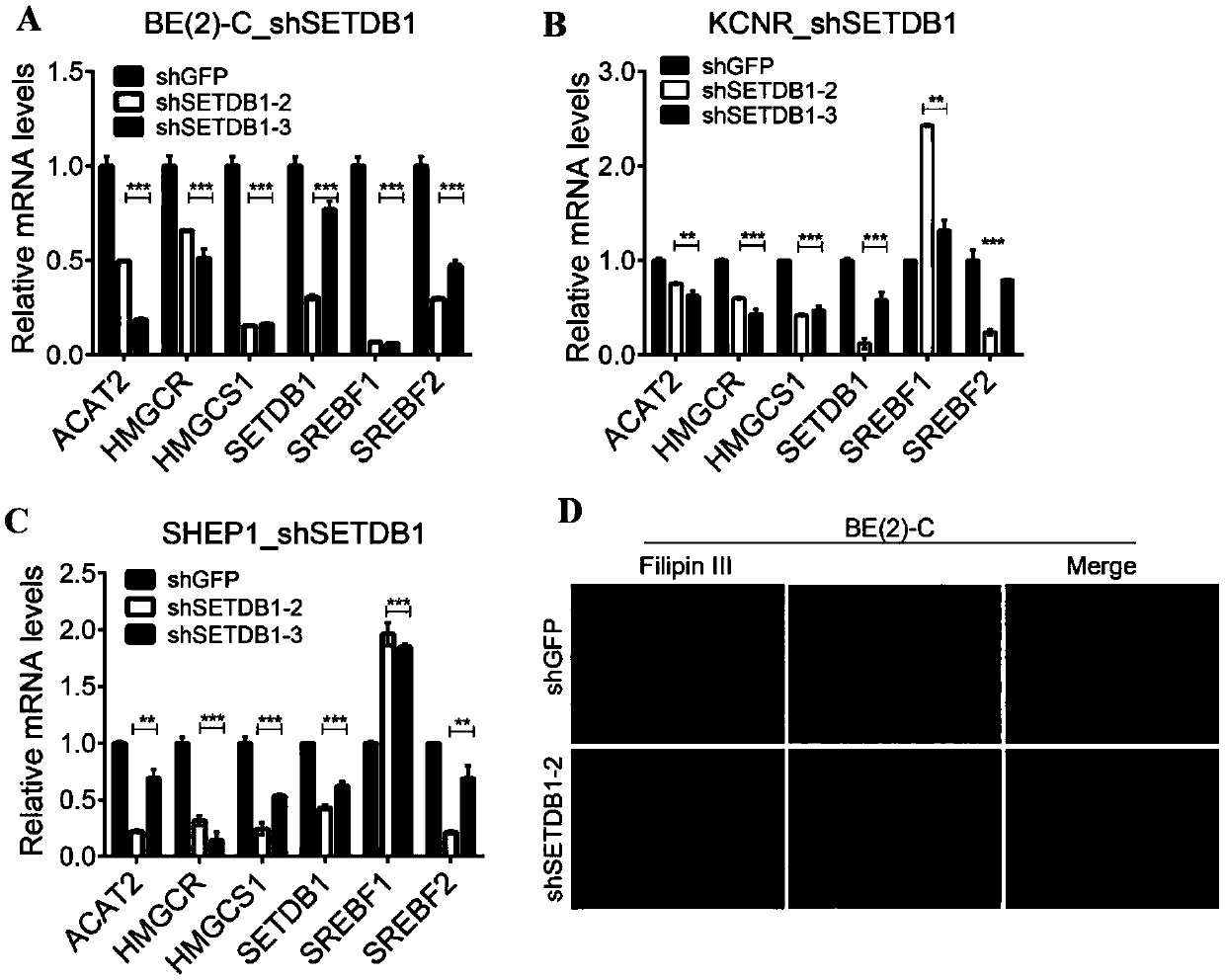
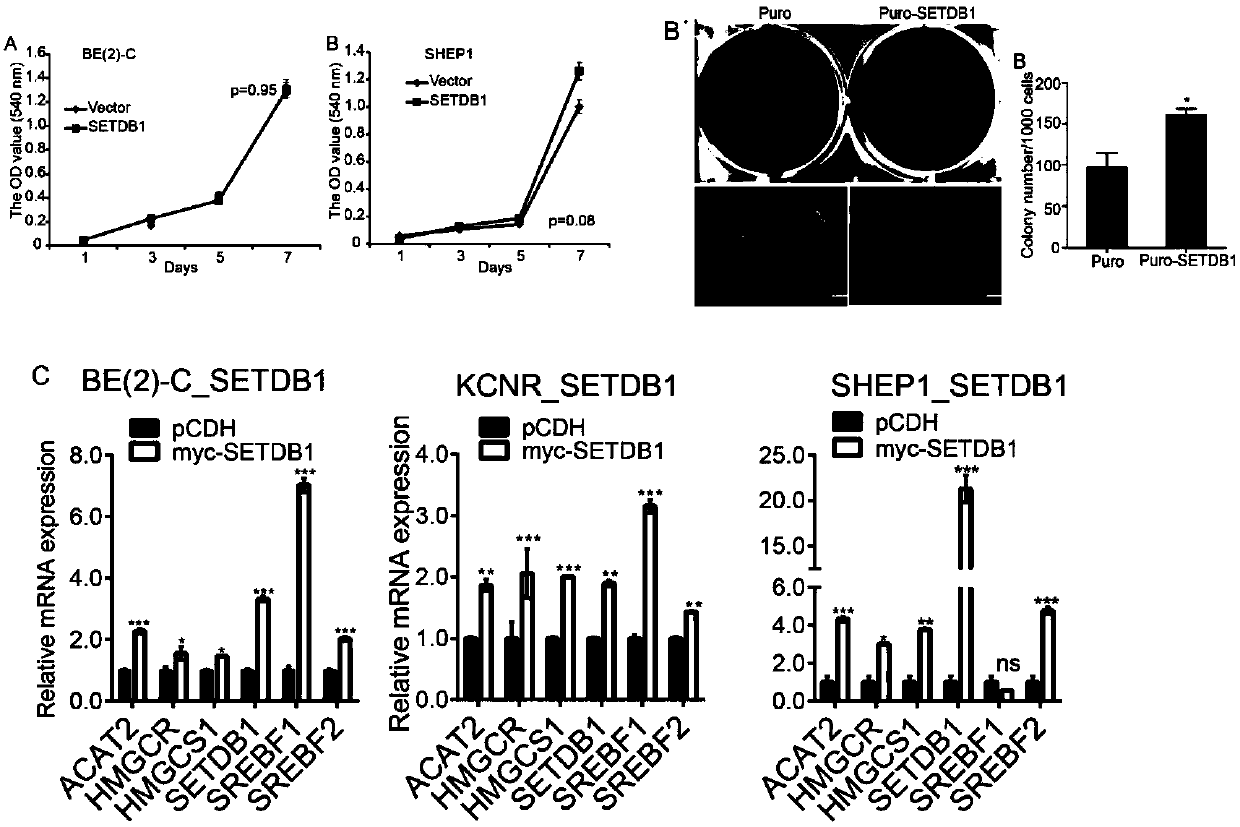
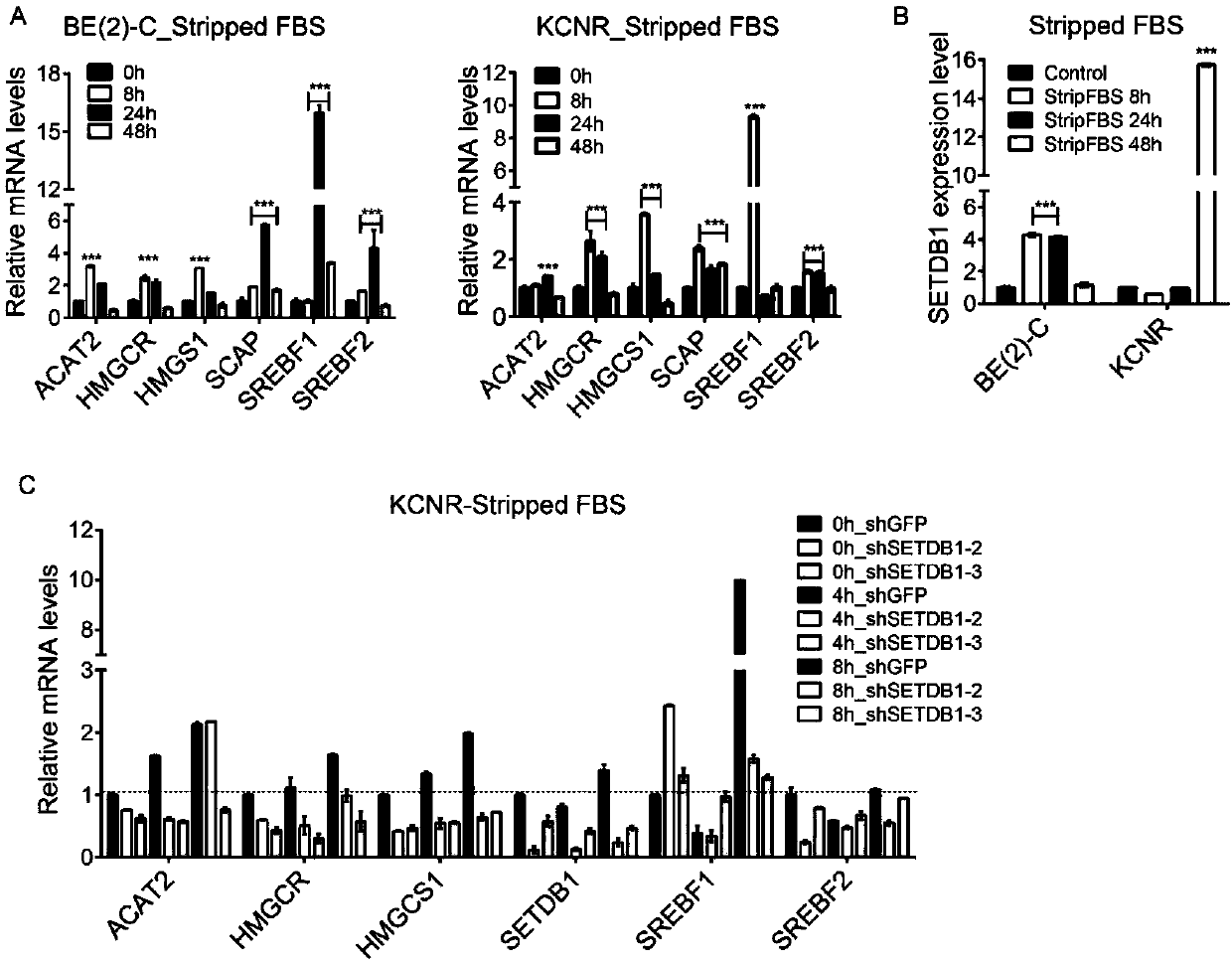
The invention discloses an application of SETDB1 to adjusting and controlling of cholesterol ways. When an SETDB1 expression is adjusted downwards, the cholesterol content can be reduced, and the expression of genes related to cholesterol ways can be adjusted downwards; when SETDB1 is in overexpression, the neuroblastoma soft agar cloning formation capacity can be improved, and the expression of genes related to the cholesterol ways can be neutralized; and SREBF2 is an activating transcription factor of the Cholesterol ways, so that when the SREBF2 is in overexpression, the expression of genesrelated to the Cholesterol ways can be notably promoted. The ways of adjusting and controlling cholesterol metabolism by the SETDB1 can be indicated to be used as a target point for diagnosis and treatment of cholesterol relevant diseases.
Owner:SOUTHWEST UNIVERSITY +1
Paratyphoid c and salmonella cholerae identification kit and preparation and use methods thereof
The invention relates to a paratyphoid c and salmonella cholerae identification kit and preparation and use methods thereof, and mainly solves the technical difficulty in quick screening and identification of paratyphoid c and salmonella cholerae in clinical infective cases and specimens such as an environment source, a food source and a livestock animal source. The paratyphoid c and salmonella cholerae identification kit comprises 1, salmonella C-group immune rabbit antiserum; 2, salmonella H-phase immune rabbit antiserum; 3, salmonella H-phase Vi immune rabbit antiserum; 4-6, dulcite, arabinose and tan sugar fermentation pipe; 7, mucic acid biochemical identification pipe; 8, potassium tartrate biochemical identification pipe; 9, sterilization mineral oil; 10, H-phase special induction soft agar; 11, induction serum H: c and H: 5 factor. The paratyphoid c and salmonella cholerae identification kit is mainly used for screening and identifying paratyphoid c and salmonella cholerae in conventional work.
Owner:上海市普陀区疾病预防控制中心 +1
Preparation method of immortalized human intervertebral disc nucleus pulposus cell system
ActiveCN102093980BImprove the level of prevention and controlHave a normal growth rateEnzymesVector-based foreign material introductionTelomeraseReverse transcriptase
The invention discloses a preparation method of an immortalized human intervertebral disc nucleus pulposus cell system. The preparation method is as follows: extrinsic human telomerase reverse transcriptase is transfected into the human nucleus pulposus cell to activate telomerase and construct immortalized human nucleus pulposus cell line through induction immortalized human nucleus pulposus cell line, normal cells not transformed cells are used, wherein the cells have normal growth rate and karyotype, have contact inhibition, anchorage dependence and other safety properties and do not have carcinogenicity and soft agar cloning capability; and as same as the germline stem cells, the cells have real immortalization, thus the standard cell line can be provided for the further research of the pathogenic mechanism of the degenerative disc disease and a new idea is provided to increase the entire control level of the degenerative disc disease.
Owner:梁伟国
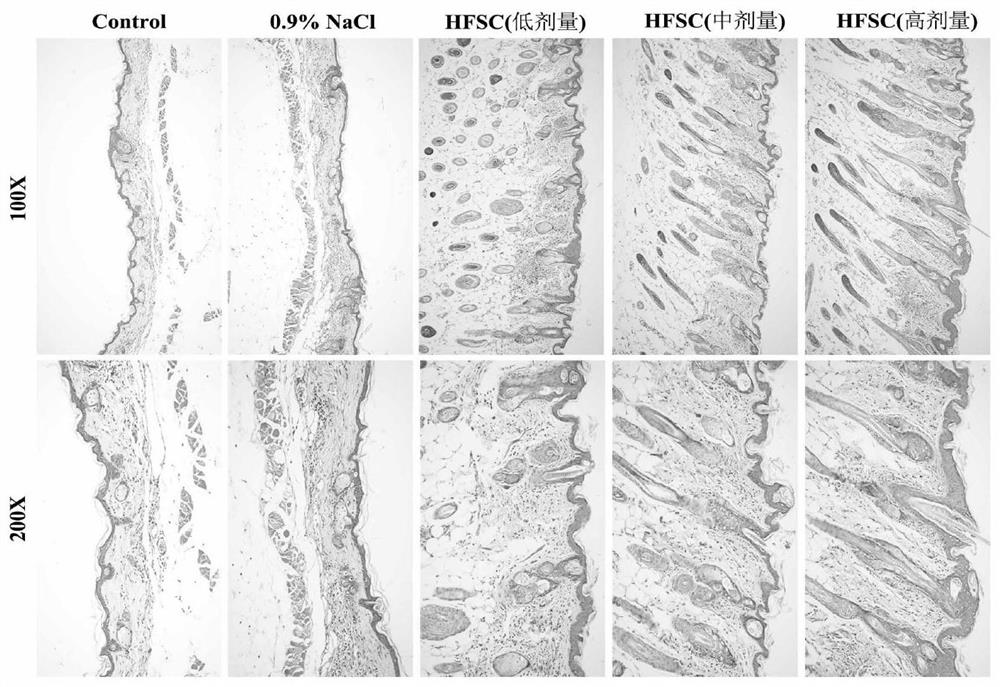
Hair follicle microarray co-culture system and application in medicine for treating pathological alopecia
ActiveCN112300982ACell dissociation methodsPeptide/protein ingredientsHair follicle stemPharmaceutical drug
The invention discloses a hair follicle microarray co-culture system and application in treating pathological alopecia. The hair follicle microarray co-culture system comprises a hair follicle stem cell suspension and damaged hair follicles to be repaired. According to the invention, a new idea for creating a microenvironment suitable for hair follicle repair by combining allogeneic normal human hair follicle stem cells with drug molecules is provided, and the hair follicle microarray co-culture system for performing in-vitro 3D co-culture on hair follicle stem cells and damaged hair folliclesby using soft agar is provided.
Owner:HUAKE TONGJI STEM CELL GENETIC ENG CO LTD
Cellulose oligosaccharide transporter as well as preparation method and application thereof
PendingCN114807201AImprove transport efficiencyPromote fermentationTransferasesMicroorganism based processesCelluloseWild type
The invention provides a cellooligosaccharide transporter as well as a preparation method and application thereof, the preparation method of the cellooligosaccharide transporter engineering strain comprises the following steps: step 1, taking a wild type SP-LacY as an initial bacteriophage, taking a visual bacteriophage-assisted continuous directed evolution system engineering bacterium containing an AP1 plasmid, an AP2 plasmid and an MP1 plasmid as a host, and preparing a cellooligosaccharide transporter engineering strain; culturing phage spots by using LB or M9 soft agar containing cellooligosaccharides as a culture medium; step 2, continuing to perform multi-round evolution culture on the obtained phage plaque, sequencing the phage plaque obtained in each round of evolution to verify the mutant accumulation trend, stopping evolution until no new mutant is formed, and finally obtaining proteins with different mutation sites; and step 3, combining different mutation sites of the protein obtained in the step 2 to construct different LacY mutant expression vectors, and transforming the LacY mutant expression vectors into host bacteria to obtain the LacY mutant. The cellooligosaccharide transporter provided by the invention can better ferment and utilize cellulose and cellooligosaccharide.
Owner:GUANGZHOU INST OF ADVANCED TECH CHINESE ACAD OF SCI
A kind of human ovarian cancer cell line and its preparation method and application
ActiveCN109929804BProliferate fastHigh selectivityMicrobiological testing/measurementMicroorganism based processesCancer cellOncology
The present invention provides a human cervical adenocarcinoma cell line, which is characterized by naming as a primary cervical adenocarcinoma drug-resistant strain W038, and is deposited in the China Center for Type Culture Collection with a preservation number being CCTCC No: C2017246. The primary cervical adenocarcinoma drug-resistant strain W038 has weak invasion ability and weak migration ability, and can be used as a double-negative control group for invasion and migration. The in-vitro soft agar colony formation test proves that the tumorigenicity proves that the cloning ability is strong, and the in-vitro susceptibility test that the strain is resistant to a variety of drugs, which is consistent with clinical features. The preparation method disclosed by the invention can purify cancer cell lines to over 99%, wherein the screened medium component can selectively kill histocyte, has high selectivity for tumor cell growth, and can increase the proliferation rate of tumor cells and shorten the culture period. The method can be used not only to purify the cervical adenocarcinoma lines, but also to purify and prepare other cancer cell lines.
Owner:TIANJIN MEDICAL UNIV CANCER INST & HOSPITAL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com