Cellulose oligosaccharide transporter as well as preparation method and application thereof
A technology of cellooligosaccharides and transport proteins, applied in the field of genetic engineering, can solve the problems of waste of resources, low utilization rate of fiber, low transfer efficiency of cellooligosaccharides, etc., and achieve the effect of improving transfer efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The present embodiment provides a preparation method of a cellobiose transporter engineered strain, which is specifically as follows:
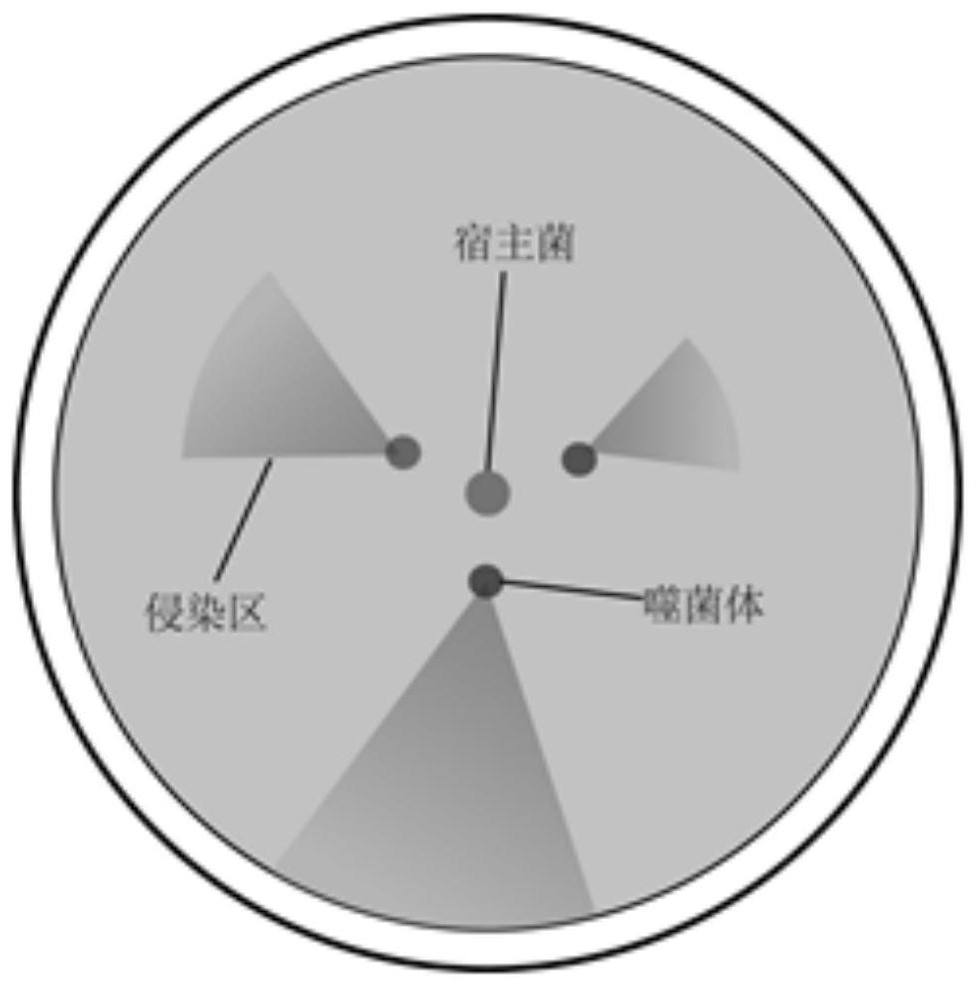
[0034] Step 1. Visualize the construction of a phage-assisted continuous directed evolution system:
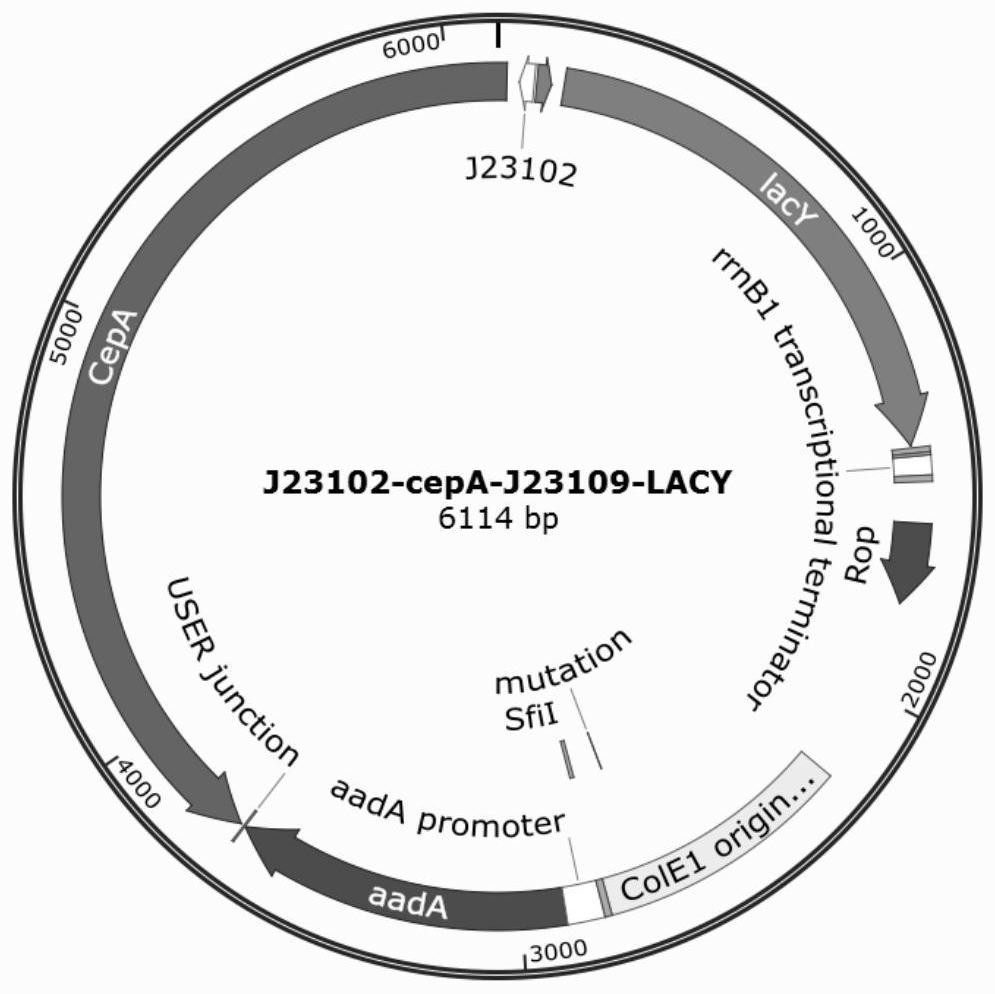
[0035] The system includes three plasmids, AP1, AP2, and MP1. The functional elements on the three plasmids can also be rearranged on two plasmids or one plasmid; the AP1 plasmid (SEQ ID NO: 2) carries the gIII gene and is promoted by the pPSP promoter. For activation, there is a recognition site CelRS (SEQ ID NO. 6) for the functional protein CelR between the pPSP promoter and the ribosome binding site RBS. In the absence of intracellular cellobiose, the expression of gIII is in a suppressed state. The AP2 plasmid (SEQ ID NO: 3) carries the CelR protein gene and the regulatory protein gene of the promoter pPSP. The mutagenic plasmid MP1 (SEQ ID NO: 4) was driven by the pPSP promoter. The host strain FM15 was constructed by itself in ...
Embodiment 2
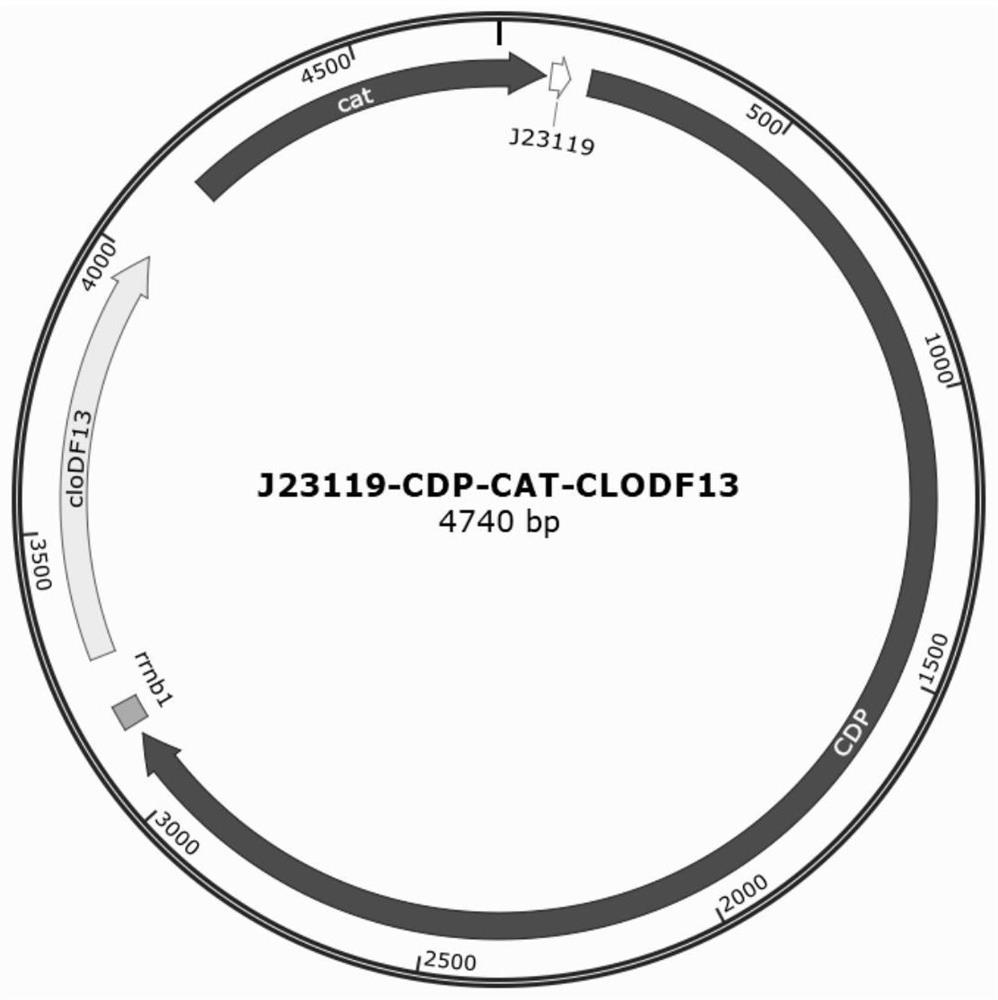
[0045] This example provides a method for preparing an engineered strain of cellotriose transporter. Steps 1 to 3 are the same as those in Example 1. The same visualized PACE system as in Example 1 is used to evolve LacY for cellotriose transport efficiency to obtain mutation sites A177V, G202V, V197A. During the construction of the engineered strain in step 4, the metabolism of cellotriose additionally requires cellooligosaccharide phosphorylase CDP to degrade it into glucose 1 phosphate and cellobiose, and cellobiose can be further degraded by cepA for bacterial utilization. Expression vector P2 (SEQ ID NO. 8) carrying CDP gene, see image 3 , the cellotriose transporter engineered strain for cellotriose metabolism can be obtained by co-transforming P1 and P2 into host S1030.
Embodiment 3
[0047] Cellobiose Metabolism Experiment of LacY Mutant Engineering Strain
[0048] Part of the engineering strains constructed in Example 1 S1030-cepA-LacYWT (wild type), S1030-cepA-LacYA177V, S1030-cepA-LacYL70H, S1030-cepA-LacYA177V / V197A, S1030-cepA-LacYA177V / G202V, S1030 -cepA-LacYA177V / N204S was cultured in M9 medium with cellobiose as the sole carbon source; the cellobiose consumption curves of different engineering strains were determined to judge the efficiency of different LacY mutants to transport cellobiose. In addition, the mutation sites G71V, A213P, and F356S were eliminated due to their relatively short appearance time and were not investigated.
[0049] from Figure 4 It can be seen from the displayed results that the sugar consumption rates of different engineered strains are: A177V / V197A>A177V, A177V / G202V, A177V / N204S>L70H>LacYWT. Therefore, the cellobiose transfer efficiencies of different engineered strains were ranked as follows: A177V / V197A>A177V, A177...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com