A kind of human ovarian cancer cell line and its preparation method and application
A technology for ovarian cancer cells and ovarian cancer, applied in the field of cell biology, can solve the problems of limited research, chemotherapy failure of chemotherapy drugs, etc., and achieve the effect of weak migration ability, stable drug resistance multiple, and weak invasion ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
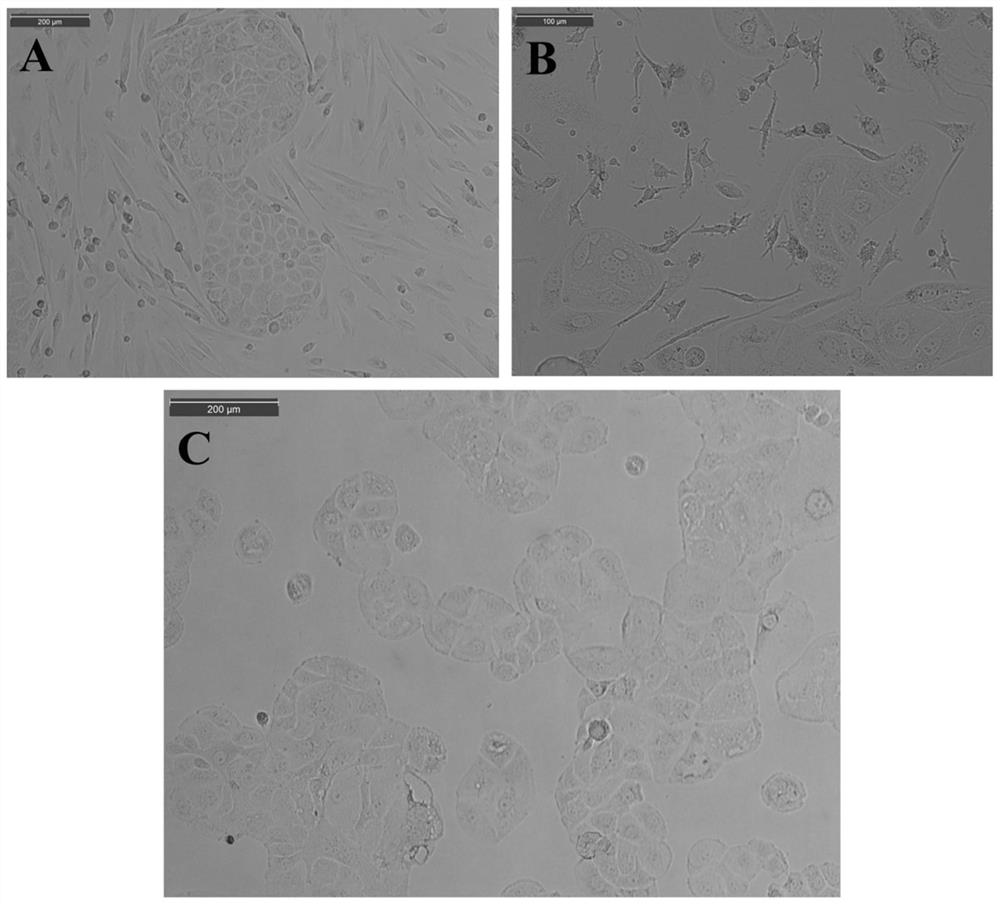
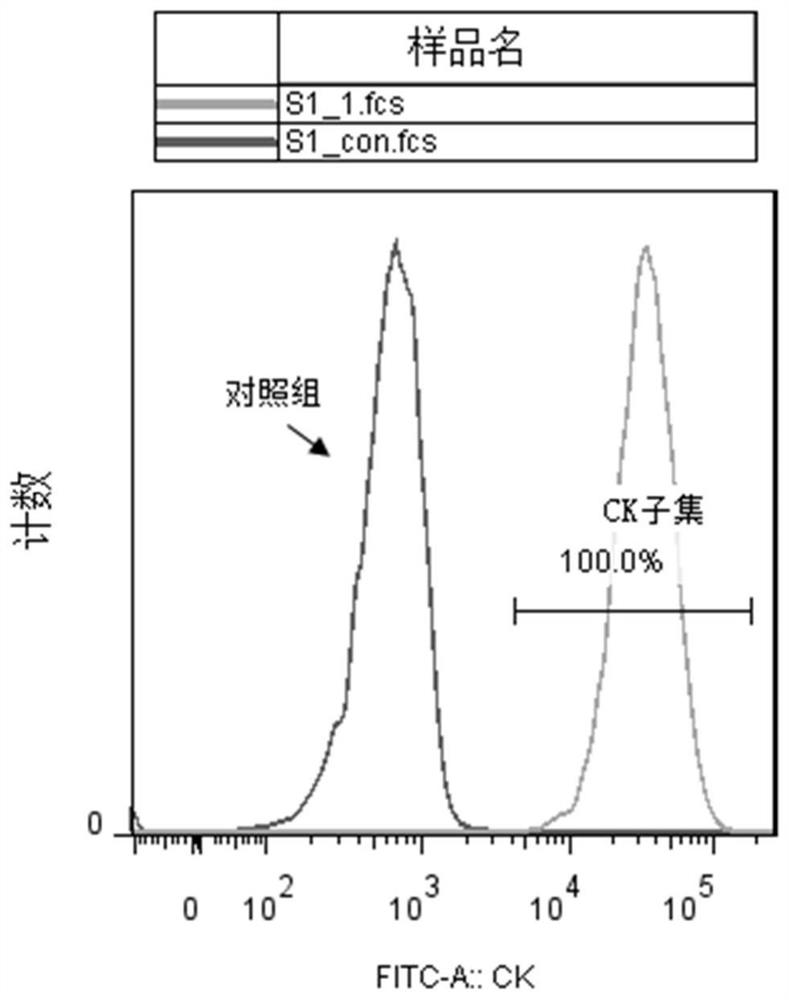
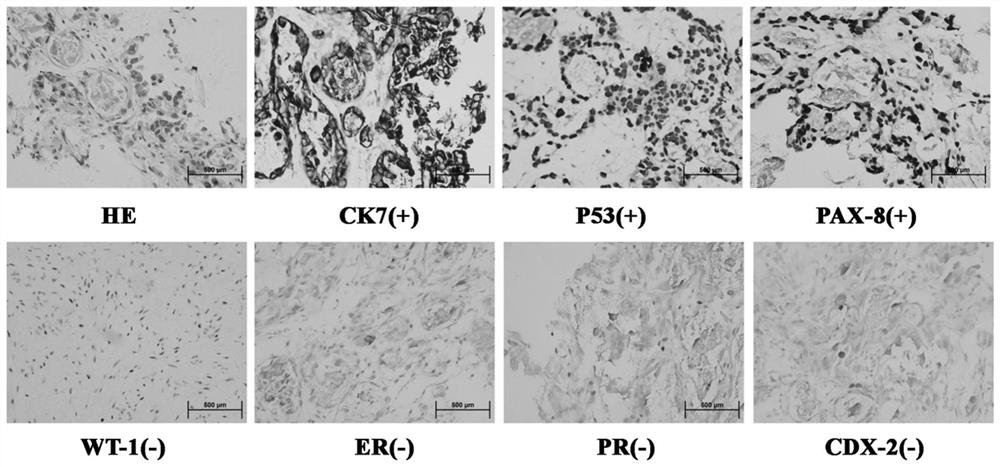
Embodiment 1
[0051] Example 1: Construction of Cell Lines
[0052] 1. Collection of Ovarian Ascites Samples
[0053] During the collection and transportation of liquid samples obtained from surgery, all reagents and instruments must be sterile, and they must be transported to the laboratory for processing within 6 hours after being separated from the body. Samples should be labeled "Ascites, peritoneal fluid, or peritoneal washings". And record the relevant information in the form. It is suggested that the patient’s name should not be recorded as the ID number, and the information on the form should be transferred to the experimental record book to ensure that the relevant clinical information is consistent with any tumor cell lines established from the sample.
[0054] 2. Pre-inoculation of primary ovarian cancer cells
[0055] 1) Under aseptic operation, transfer the ascites to a 50mL centrifuge tube with a sterile conical bottom, and centrifuge at 1000rpm at 4°C for 7min;
[0056] 2...
Embodiment 2
[0069] Example 2: Construction of Cell Lines
[0070] 1. Collection of Ovarian Ascites Samples
[0071] During the collection and transportation of liquid samples obtained from surgery, all reagents and instruments must be sterile, and they must be transported to the laboratory for processing within 6 hours after being separated from the body. Samples should be labeled "Ascites, peritoneal fluid, or peritoneal washings". And record the relevant information in the form. It is suggested that the patient’s name should not be recorded as the ID number, and the information on the form should be transferred to the experimental record book to ensure that the relevant clinical information is consistent with any tumor cell lines established from the sample.
[0072] 2. Pre-inoculation of primary ovarian cancer cells
[0073] 1) Under aseptic operation, transfer the ascites to a 50mL centrifuge tube with a sterile conical bottom, and centrifuge at 1000rpm at 4°C for 7min;
[0074] 2...
Embodiment 3
[0094] Example 3: Subculture and cryopreservation of cell lines
[0095] 1. Change the liquid
[0096] Inoculate the primary culture and change the medium with fresh screening medium every 3-4 days. When changing the medium, use a sterile Pasteur pipette to suck away the old medium, and then replace it with a new straw to add new complete medium.
[0097] 2. Avoid cross-contamination
[0098]To reduce or avoid the possibility of contamination of newly cultured cell lines with cells from other sources, use the following precautions:
[0099] If conditions permit, when the primary cultured cell line is established, the laboratory will not cultivate other established cell lines. If conditions do not permit, try to ensure that the newly established primary cultured cell line is cultured in an independent ultra-clean bench and incubator. The medium used is separately packed in a sterile container and marked separately. Each culture operation is carried out in a cell culture ultra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com