Connective tissue growth factor fragments and methods and uses thereof
a technology of connective tissue and growth factor, which is applied in the field of growth factor fragments, can solve the problems that ctgf alone cannot induce this property in fibroblasts, and achieve the effects of increasing the activity of ctgf, suppressing or suppressing the mitogenic activity, and increasing the mitogenic activity of ctg
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
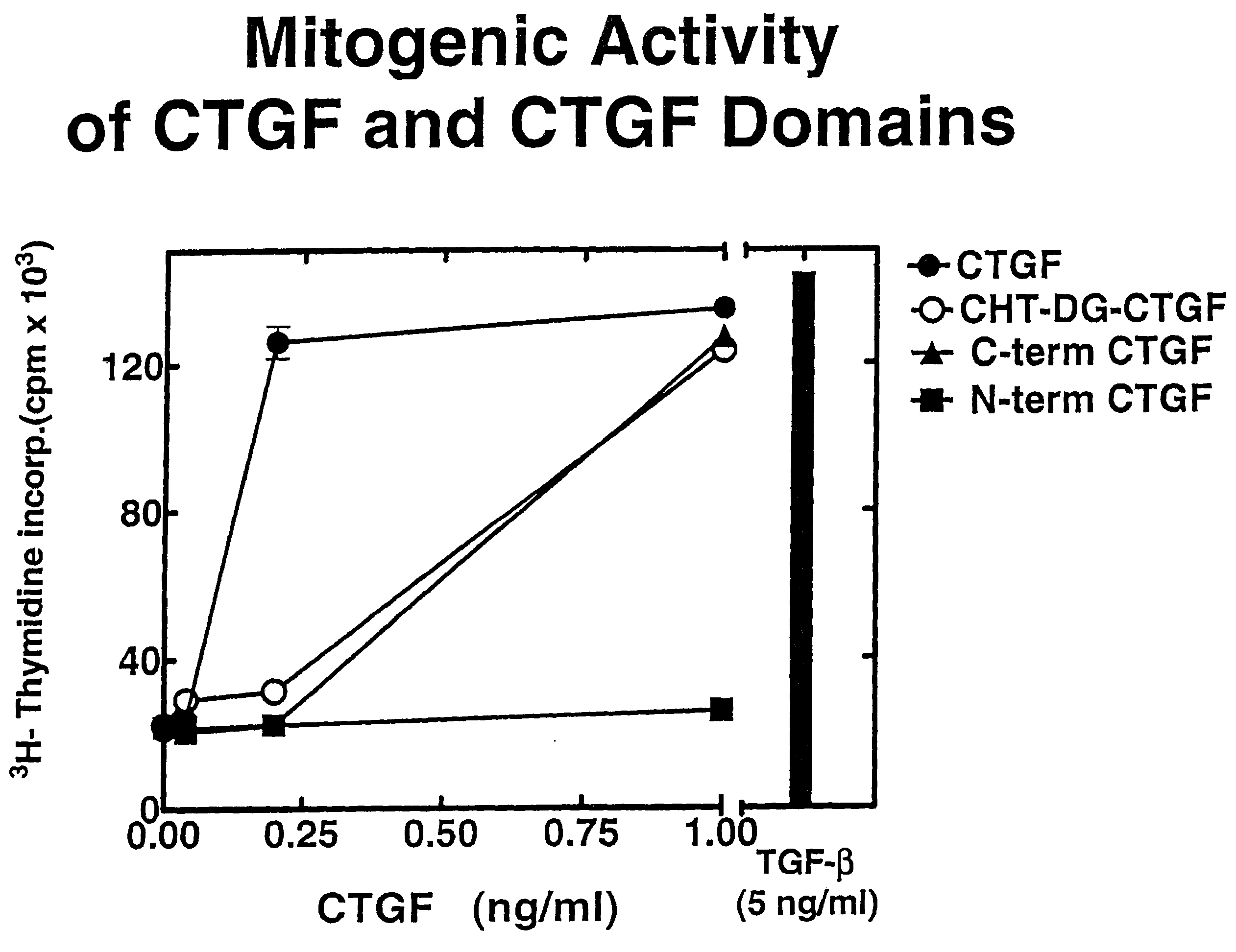
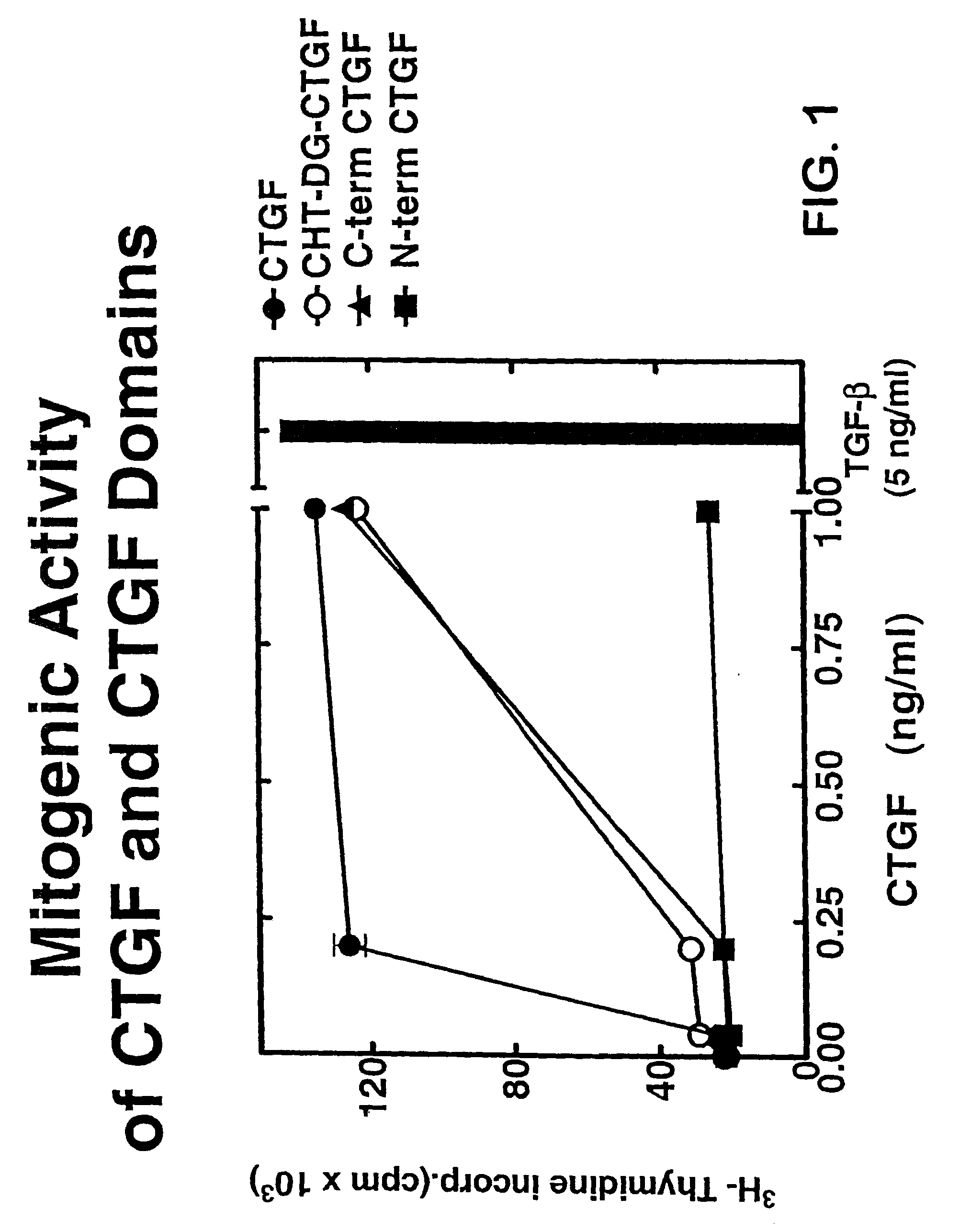
CTGF Fragments Stimulate DNA Synthesis
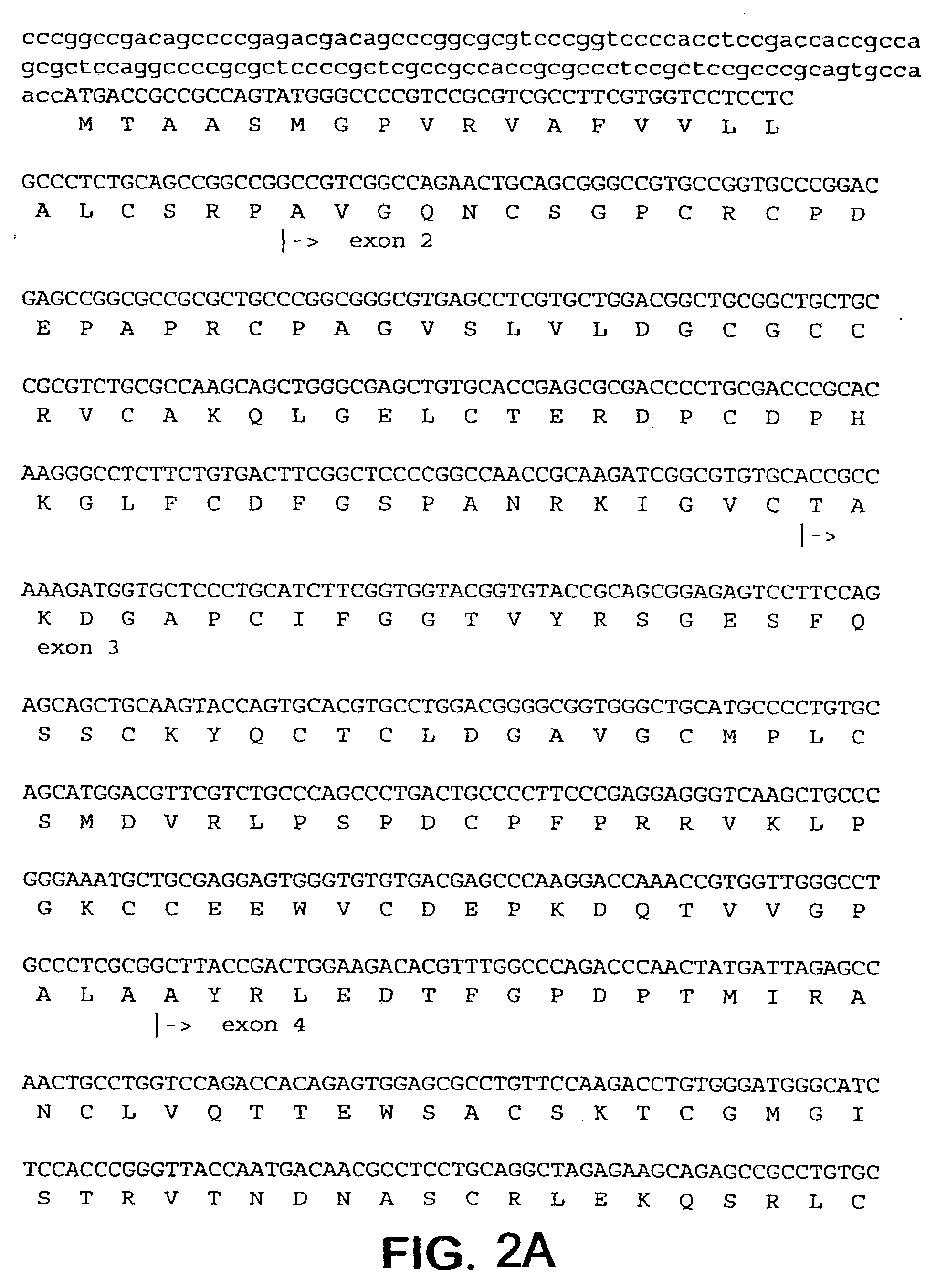
[0159]To prepare CTGF fragments, human recombinant CTGF (full length) was digested by chymotrypsin to render one CTGF fragment. Recombinant CTGF fragments were also produced by expressing either or both exon 4 and exon 5 of CTGF. A continuous line of cultured normal rat kidney (NRK) fibroblasts, designated as clone NRK-49F, were obtained from the American Type Culture Collection (ATCC) to produce cell cultures. Human foreskin fibroblasts were established from explant cultures. Cell cultures were maintained in Dulbecco's modified eagle media (DME) containing 2.5% fetal bovine serum and 2.5% Nu-Serum I (Collaborative Biomedical Products, Bedford, Mass.) and passaged prior to confluence.
[0160]To examine the role of CTGF fragments in mitogenesis and DNA synthesis, growth arrested monolayers of NRK and human foreskin fibroblasts were prepared by seeding 10,000 cells / well in 48 well plates, and allowing the cells to grow to confluence in 5 to 7 days i...
example 2
Neutralizing Anti-CTGF Antibodies Block TGF-β Induced DNA Synthesis, Collagen Synthesis, and Myofibroblast Induction
[0162]Specific anti-CTGF antibodies were raised against biologically active recombinant human CTGF produced in a baculovirus expression system using methods known in the art. The antibodies were prepared in goats and tested for neutralization activity of CTGF directly or on TGF-induced DNA or collagen synthesis in NRK fibroblasts. The goat antibodies exhibited activity in the assays for neutralization of TGF-β action. In these assays, the goat anti-CTGF antibodies were able to block DNA synthesis as demonstrated in FIG. 4, collagen synthesis, and myofibroblast formation induced by TGF-β. It was noted that the amount of antibody required to block collagen synthesis was significantly less than the amount needed to block DNA synthesis. Both western blot assay, and competition ELISA assays indicated that most of the antibodies in this preparation were directed against the ...
example 3
Anti-CTGF Antibodies Specific for the C-Terminal Domain of CTGF Selectively Block DNA Synthesis
[0163]Domain specific anti-CTGF antibodies were prepared by affinity chromatography using purified C-terminal or N-terminal CTGF domains. These domains were prepared from intact CTGF by limited digestion with chymotrypsin. The domains were separated from each other by affinity chromatography on heparin sepharose. The N-terminal domain does not bind to heparin whereas the C-terminal domain of CTGF contains the heparin binding activity and is retained on the heparin sepharose. These domains were pure, having less than 0.1% contamination with intact CTGF based on western blot analysis. The individual domains were then coupled to Affigel 10 at a concentration of approximately 0.5 mg / ml of gel. Total anti-CTGF IgG (goat) was then absorbed to the affinity resin, and the specifically bound antibodies were eluted. These antibodies were then tested in western blots to determine the specificity of t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com