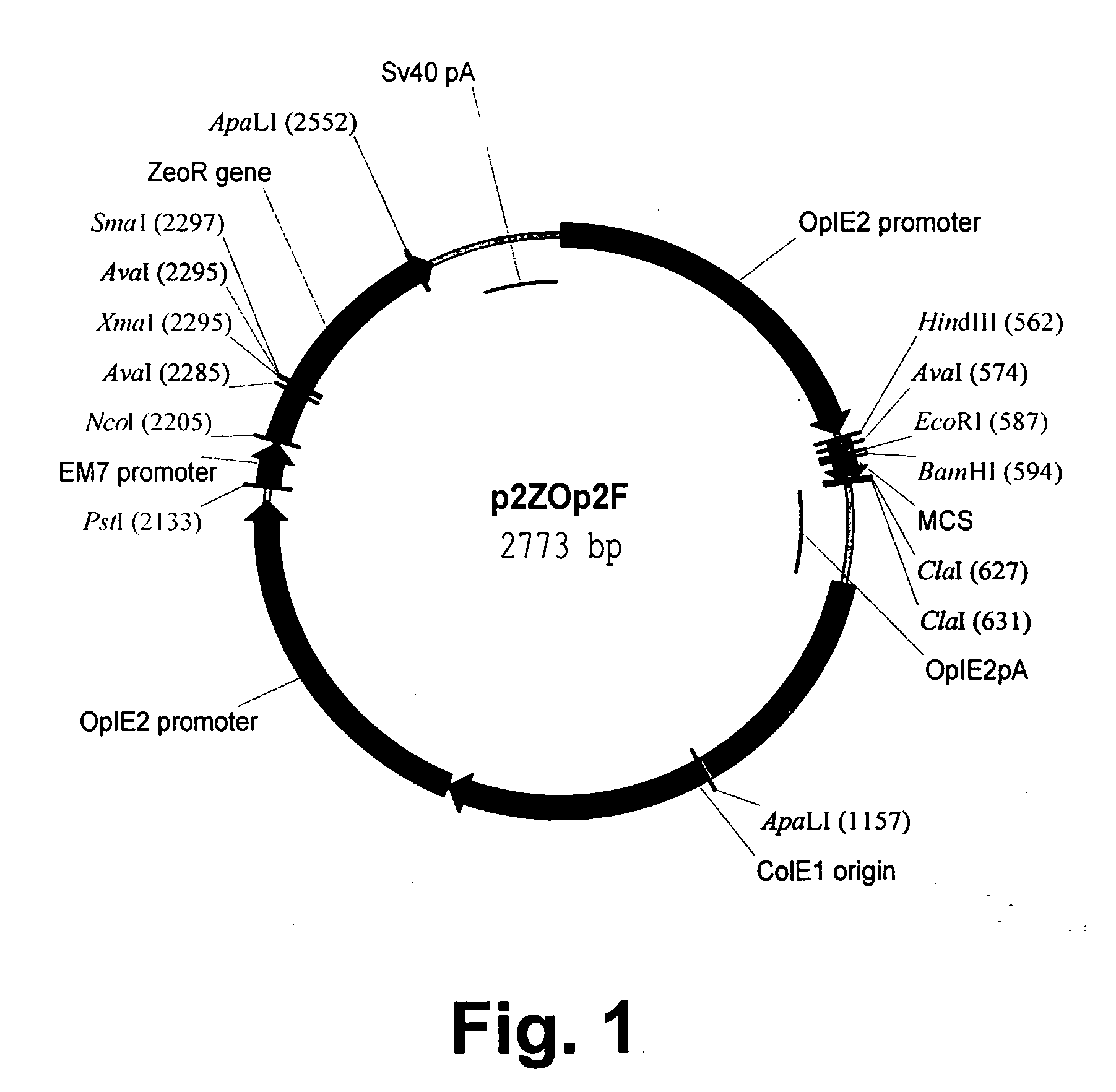
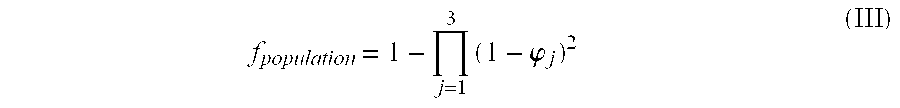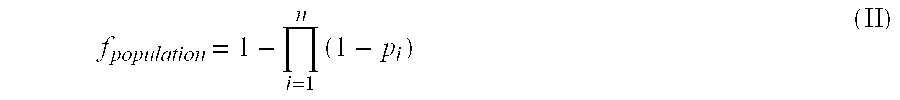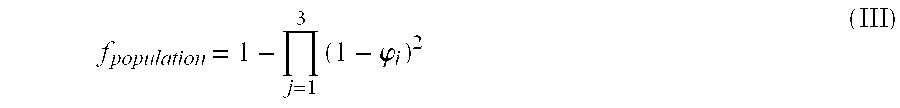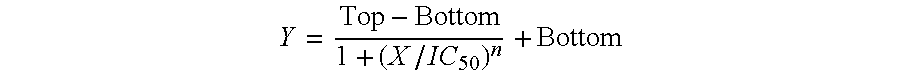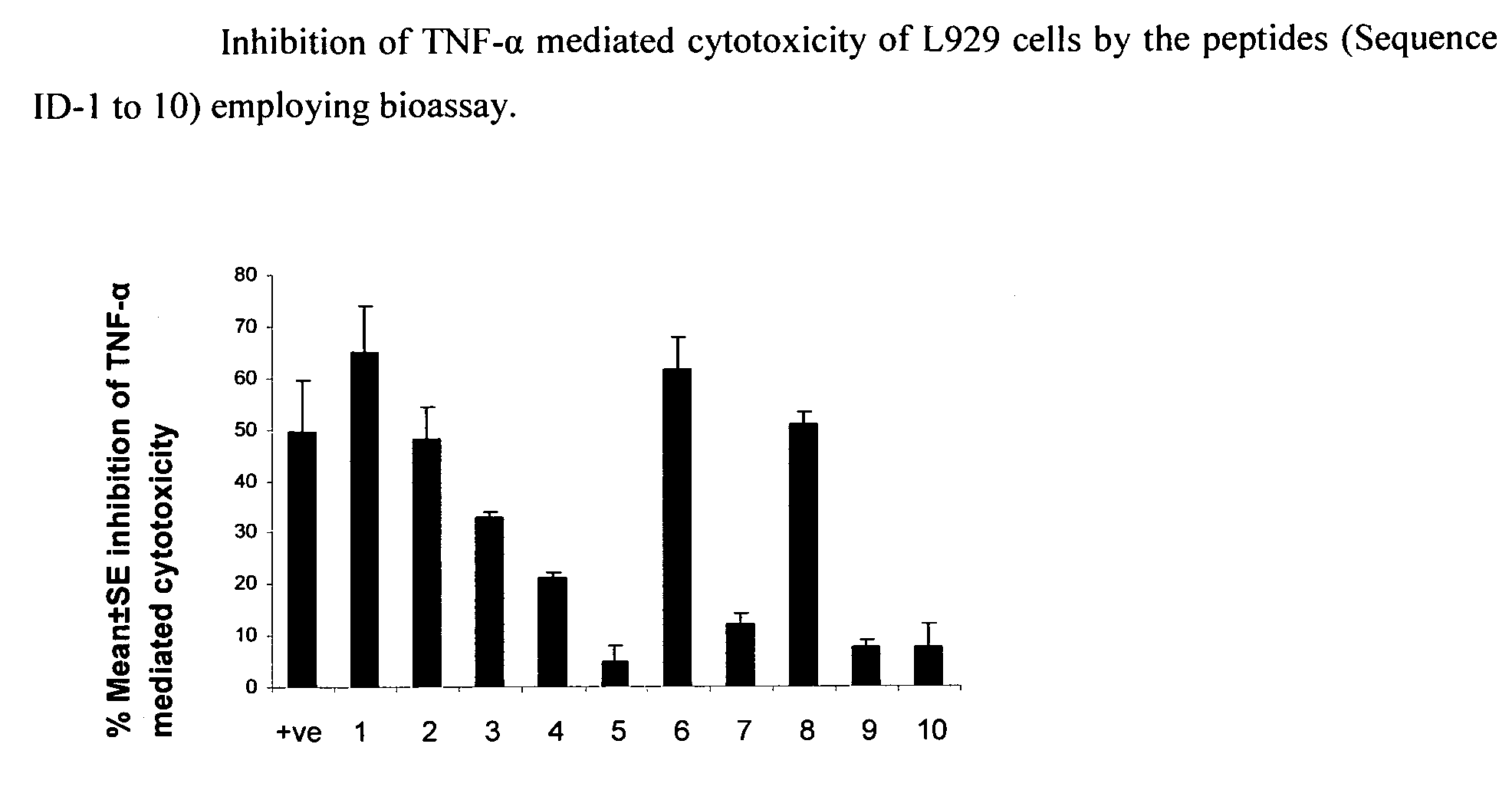Patents
Literature
52 results about "Tumour necrosis factor alpha" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tumor necrosis factor (TNF, tumor necrosis factor alpha, TNFα, cachexin, or cachectin) is a cell signaling protein (cytokine) involved in systemic inflammation and is one of the cytokines that make up the acute phase reaction.
Method and composition for treatment of renal failure with antibodies and their equivalents as partial or complete replacement for dialysis
InactiveUS7504106B2Lower Level RequirementsSlow onsetBiocidePeptide/protein ingredientsInterleukin 6Creatinine rise
A method for treating patients with renal failure includes administering to them an effective amount of antibody or of a functional equivalent thereof to at least two of urea, creatinine, tumor necrosis factor alpha, interferon gamma, interleukin 6 and interleukin 1 beta. Soluble cytokine receptors also can be employed. The method can be used as a supplement to or as partial or complete replacement for dialysis. A pharmaceutical composition includes antibody or functional equivalent thereof to urea, creatinine, or both; antibody, functional equivalent or soluble cytokine receptor to tumor necrosis factor alpha, interferon gamma, interleukin 6, interleukin 1 beta or any combination thereof The composition can be included in a kit.
Owner:SKURKOVICH BORIS +2
Small molecule inhibitors of necroptosis
The invention features a series of heterocyclic derivatives that inhibit tumor necrosis factor alpha (TNF-α) induced necroptosis. The heterocyclic compounds of the invention are described by Formulas (I)-(VIII) and by Compounds (I)-(I), (13)-(26), (27)-(33), (48)-(57), and (58)-(70). These necrostatins are shown to inhibit TNF-α induced necroptosis in FADD-deficient variant of human Jurkat T cells. The invention further features pharmaceutical compositions featuring necrostatins. The compounds and compositions of the invention may also be used to treat disorders where necroptosis is likely to play a substantial role.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Tumor necrosis factor alpha
InactiveUS20070037164A1Raise the possibilityDelay and reduce and preventSugar derivativesMicrobiological testing/measurementFactor iiTumour necrosis factor alpha
The present disclosure describes the use of genetic variance information for genes involved in inflammatory or immunologic disease, disorder, or dysfunction. The variance information is indicative of the expected response of a patient to a method of treatment. Methods of determining relevant variance information and additional methods of using such variance information are also described.
Owner:NUVELO INC
Dermatological pharmaceutical composition for the treatment of skin inflammation diseases, such as dermatitis, atopic dermatitis, vitiligo, alopecia areata, acne, psoriasis, pruritus or combinations of same
InactiveUS20110129546A1Improve antioxidant capacityGood anti-inflammatory effectBiocideAntimycoticsDiseaseIsoflavones
The invention relates to a dermatological pharmaceutical composition for the treatment of skin inflammation diseases, such as dermatitis, atopic dermatitis, vitiligo, alopecia areata, acne, psoriasis and pruritus. The invention comprises a base anti-inflammatory agent, such as indometacin; one or more optional active ingredients selected alternatively from among at least a corticoid and an antibiotic; and a combination of topical antioxidants used to potentiate the anti-inflammatory effect, selected from among green tea, lipoic acid, curcumin, ascorbyl palmitate, Coenzyme Q10, resveratrol, Pycnogenol™, L-camosine, taurine, vitamin E, vitamin C, papaya extract, isoflavones, manganese, lycopene and quercetin. At least one of the topical antioxidants is a peroxisome proliferator-activated receptor-gamma (PPAR-γ) activator. The invention also includes at least one antioxidant substance with an antiproliferative effect on keratonocytes, e.g. manganese, and at least one substance that blocks tumour necrosis factor-alpha (TNF-α) or other cytokines that provoke the acute phase of the inflammatory reaction, also with an antiproliferative effect, e.g. pentoxifylline.
Owner:UMBERT MILL IGNACIO
Tumour necrosis factor binding ligands
InactiveUS20040214993A2Peptide/protein ingredientsAntibody mimetics/scaffoldsAntiendomysial antibodiesSingle-Chain Antibodies
Abstract of the Disclosure The present invention relates to ligands which bind to human tumour necrosis factor alpha (TNF) in a manner such that upon binding of these ligands to TNF the biological activity of TNF is modified. In preferred forms the ligand binds to TNF in a manner such that the induction of endothelial procoagulant activity of the TNF is inhibited; the binding of TNF to receptors on endothelial cells is inhibited; the induction of fibrin deposition in the tumour and tumour regression activities of the TNF are enhanced; and-the cytotoxicity and receptor binding activities of the TNF are unaffected or enhanced on tumour cells. The ligand is preferably an antibody, F(ab) fragment, single domain antibody (dABs) single chain antibody or a serum binding protein. It is preferred, however, that the ligand is a monoclonal antibody or F(ab) fragment thereof.
Owner:PEPTECH
Novel immunogenic mimetics of multimer proteins
InactiveUS20030185845A1High yieldLow toxicityCell receptors/surface-antigens/surface-determinantsAntibody mimetics/scaffoldsInterleukin 5ADAMTS Proteins
The present invention relatas to novel immunogenic variants of multimeric proteins such as immunogenic variants of interleukin 5 (IL5) and tumour necrosis factor alpha (TNF, TNFalpha). The variants are, besides from being immunogenic in the autologous host, also highly similar to the native 3D structure of the proteins from which they are derived. Certain variants are monomeric mimics of the multimers, where peptide linkers (inert or T helper epitope containing) ensure a spatial organisation of the monomomer units that facilitate correct folding. A subset of variants are monomer TNFalpha variants that exhibit a superior capability of assembling into multimers with a high structural similarity to the native protein. Also disclosed are methods of treatment and production of the variants as well as DNA fragments, vectors, and host cells.
Owner:PHARMEXA
Application of dihydromyricetin in preparation of liver regeneration medicine
InactiveCN103751173APromote regrowthOrganic active ingredientsDigestive systemDismutaseAcute hepatic failure
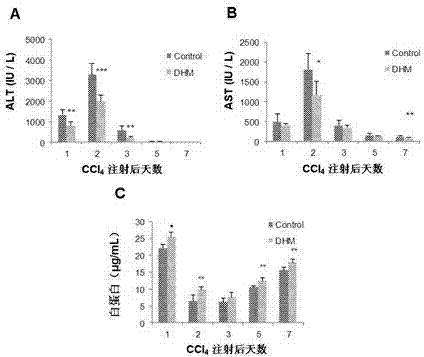
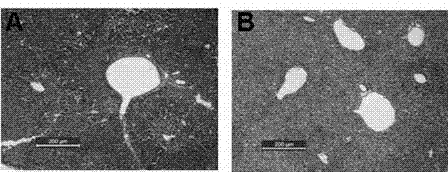
The invention relates to the biomedical field, and particularly relates to application of dihydromyricetin in preparation of a liver treatment and regeneration promotion medicine. Dihydromyricetin shows relatively high oxidation resistance and characteristic on promoting cell growth as a flavonoid compound. The test shows that dihydromyricetin can effectively relieve liver tissue necrosis caused by acute hepatic failure and can promote hepatocyte proliferation, so as to obviously raise the survival rate of a model mouse suffering from acute hepatic failure; dihydromyricetin also can obviously improve the activity of superoxide dismutase in tissue and serum, increase the content of serum protein, and lower down the levels of glutamic oxalacetic transaminase, glutamic-pyruvic transaminase and tumor necrosis factor alpha.
Owner:HOSPITAL AFFILIATED TO GUANDONG MEDICAL COLLEGE
Immunogenic mimetics of multimer proteins with promiscuous T cell epitope inserts
InactiveUS20040258660A1High expressionImprove purification effectPeptide/protein ingredientsAntibody mimetics/scaffoldsInterleukin 5ADAMTS Proteins
The present invention relatas to novel immunogenic variants of multimeric proteins such as immunogenic variants of interleukin 5 (IL5) and tumour necrosis factor alpha (TNF, TNFalpha). The variants are, besides from being immunogenic in the autologous host, also highly similar to the native 3D structure of the proteins from which they are derived. Certain variants are monomeric mimics of the multimers, where peptide linkers (inert or T helper epitope containing) ensure a spatial organisation of the monomomer units that facilitate correct folding. A subset of variants are monomer TNFalpha variants that exhibit a superior capability of assembling into multimers with a high structural similarity to the native protein. Also disclosed are methods of treatment and production of the variants as well as DNA fragments, vectors, and host cells.
Owner:PHARMEXA
Dermatological pharmaceutical composition for the treatment of skin inflammation diseases, such as dermatitis, atopic dermatitis, vitiligo, alopecia areata, acne, psoriasis, pruritus or combinations of same
The invention relates to a dermatological pharmaceutical composition for the treatment of skin inflammation diseases, such as dermatitis, atopic dermatitis, vitiligo, alopecia areata, acne, psoriasis and pruritus. The invention comprises a basic anti-inflammatory agent, such as indometacin; one or more optional active principles selected alternatively from among at least a corticoid and an antibiotic; and a combination of topical antioxidants used to potentiate the anti-inflammatory effect, selected from among green tea, lipoic acid, curcumin, ascorbyl palmitate, Coenzyme Q10, resveratrol, Pycnogenol TM , L-camosine, taurine, vitamin E, vitamin C, papaya extract, isoflavones, manganese, lycopene and quercetin. At least one of the topical antioxidants is a peroxisome proliferator-activated receptor-gamma (PPAR- gamma) activator. The invention also includes at least one antioxidant substance with an antiproliferative effect on keratonocytes, e.g. manganese, and at least one substance that blocks tumour necrosis factor-alpha (TNF- alpha) or other cytokines that provoke the acute phase of the inflammatory reaction, also with an antiproliferative effect, e.g. pentoxifylline.
Owner:伊格纳西奥安贝尔特米列特
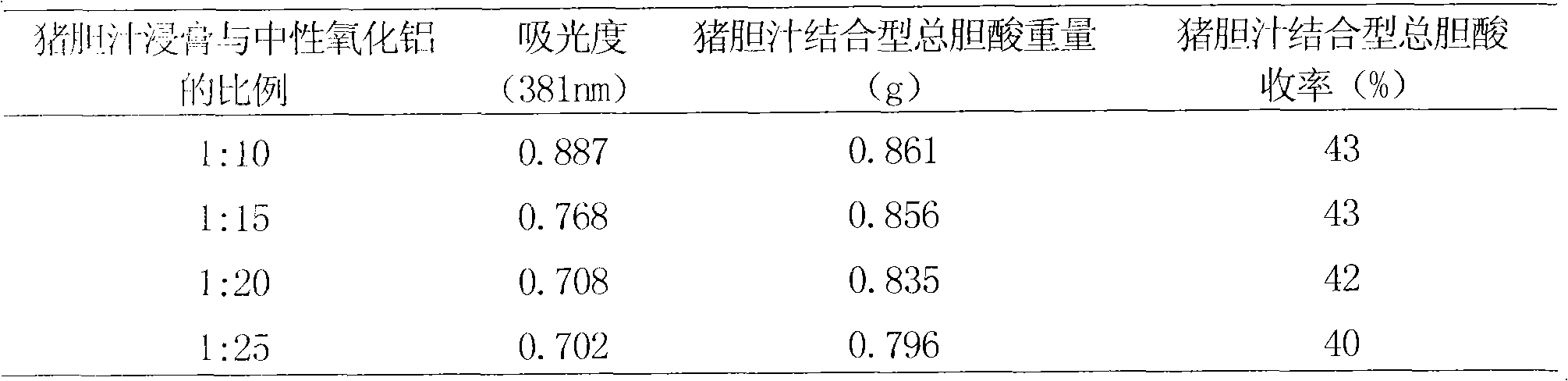
Method for preparing pig-bile-combination-type total bile acid and application thereof
ActiveCN102178695AInhibition of secretionAvoid damageDigestive systemUnknown materialsCholic acidBile Juice
The invention relates to a method for preparing pig-bile-combination-type total bile acid. The method comprises the steps of extracting by utilizing ethanol, separating and purifying and drying. Proved by medicinal effectiveness tests for treating colonitis, the pig-bile-combination-type total bile acid prepared by utilizing the method provided by the invention has an inhibitory action on the increase of myeloperoxidase activity and tumor necrosis factor alpha content of model mice and has the advantages of inhibiting the secretion of proinflammatory cytokine, regulating the immune response, effectively blocking the damage of the immunoreactivity to an organism and achieving the purpose of treating colonic inflammation. The pig-bile-combination-type total bile acid prepared by utilizing the method provided by the invention can be adopted as a raw material to be made into various commonly-used preparation types such as tablets, capsules, powders, pills, granules, enemas and the like.
Owner:NORTHWEST UNIV(CN)
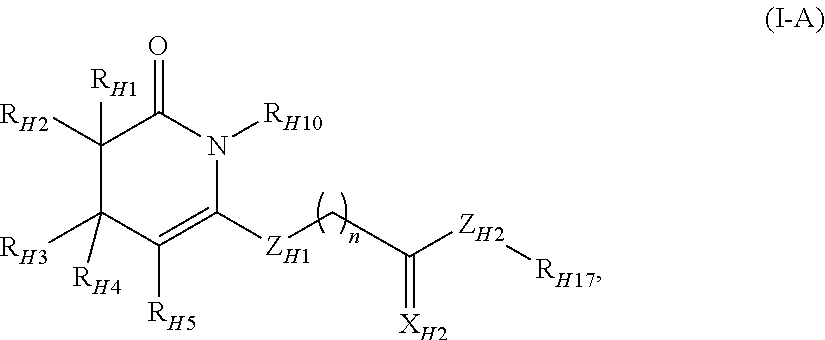
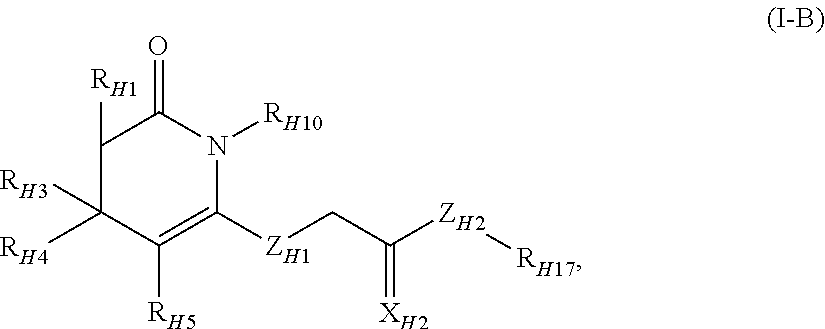
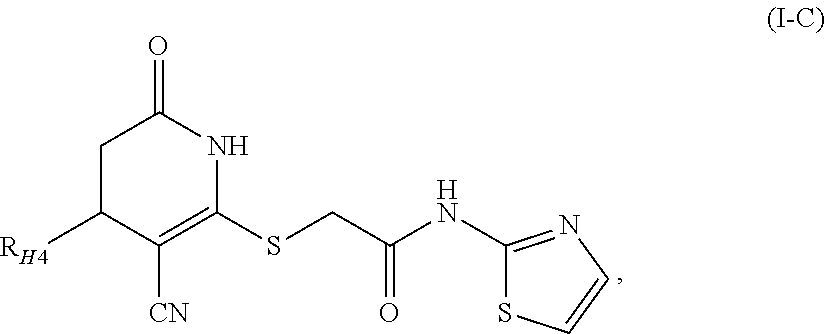
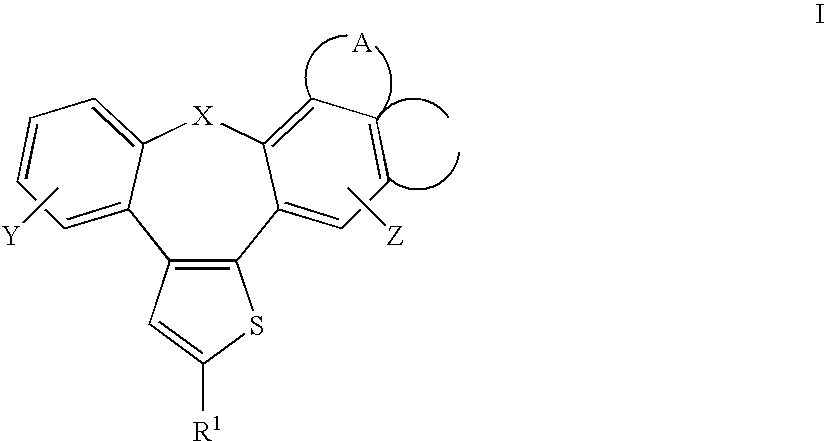
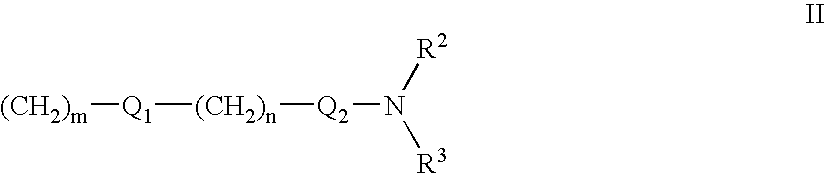
Fused Tricyclic Compounds as Inhibitors of Tumor Necrosis Factor-Alpha
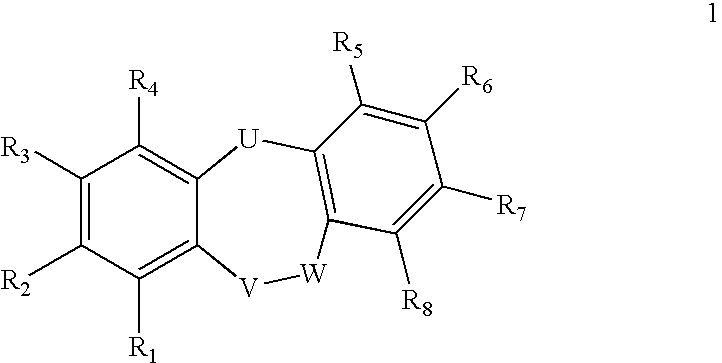
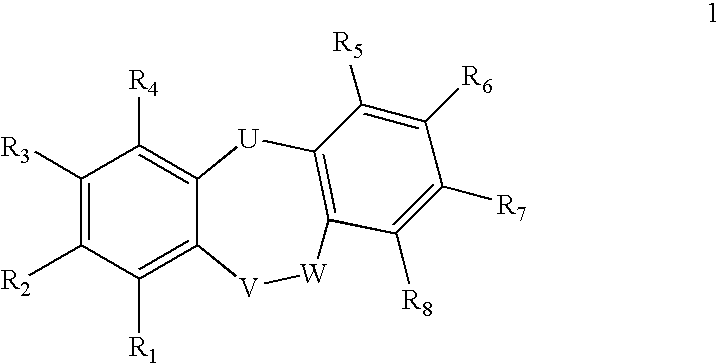
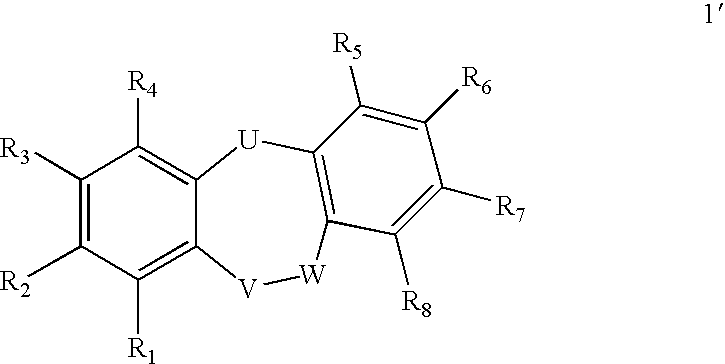
Compounds of formula 1: are disclosed, wherein V is CH2; W is S(O)m; m is the integer 0, 1 or 2; U is O, C(O), CR13R14 or NR15; where R13 is H, alkyl; R14 is H, OH, OR13 or OCOR13; R15 is H, alkyl, cycloalkyl, alkenyl, C(O)R13, C(O)OR13 or alkylaminocarbonyl; R1, R2, R3, R4, R5, R6, R7 and R8 are as defined herein. These compounds are inhibitors of tumor necrosis factor-alpha (TNF-α) and are useful as medicaments for the treatment and prevention of disorders caused by increased TNF-α activity, in particular inflammations.
Owner:PIRAMAL ENTERPRISES LTD
Topical administration of therapeutic agents and oligonucleotide formulations
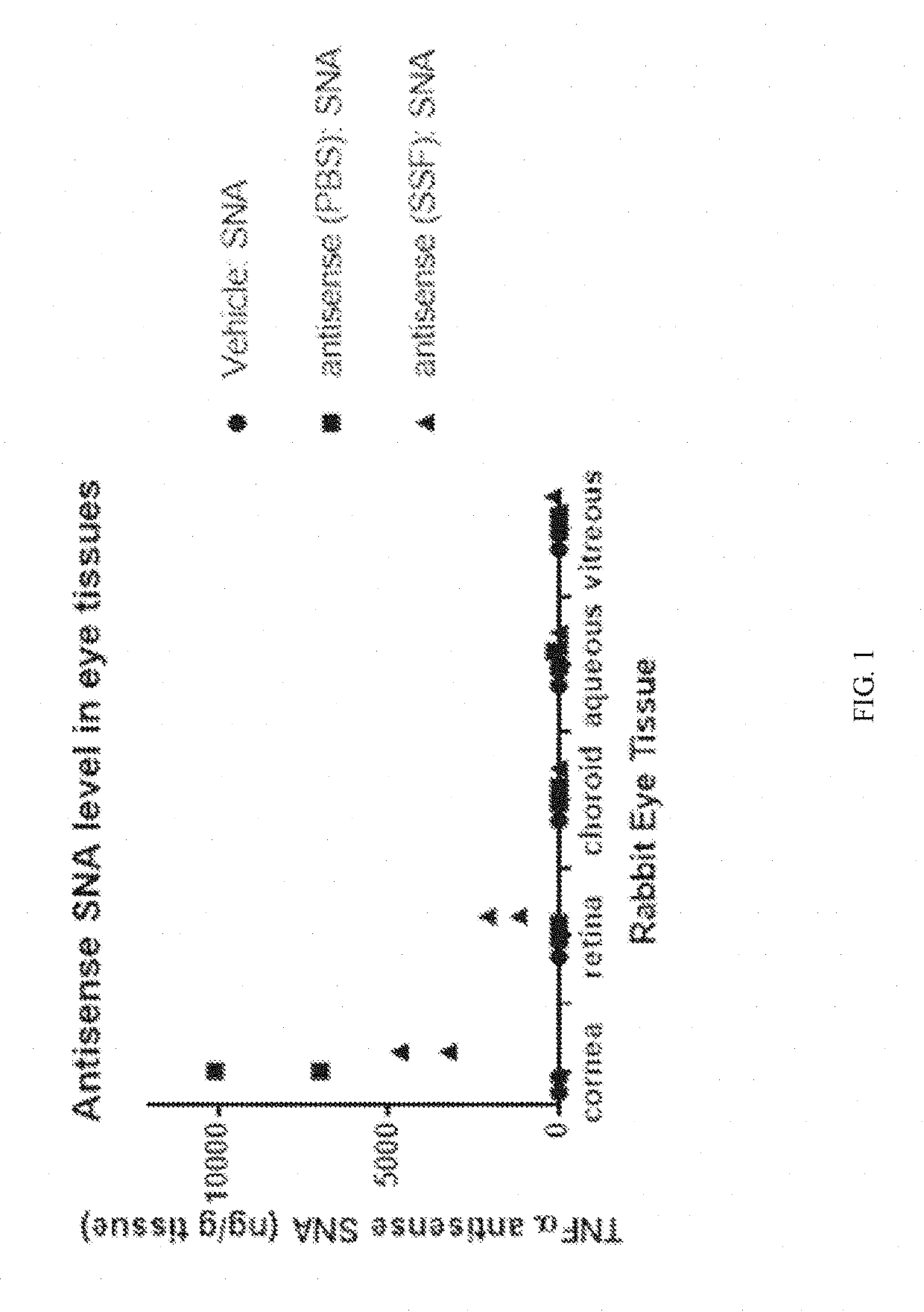
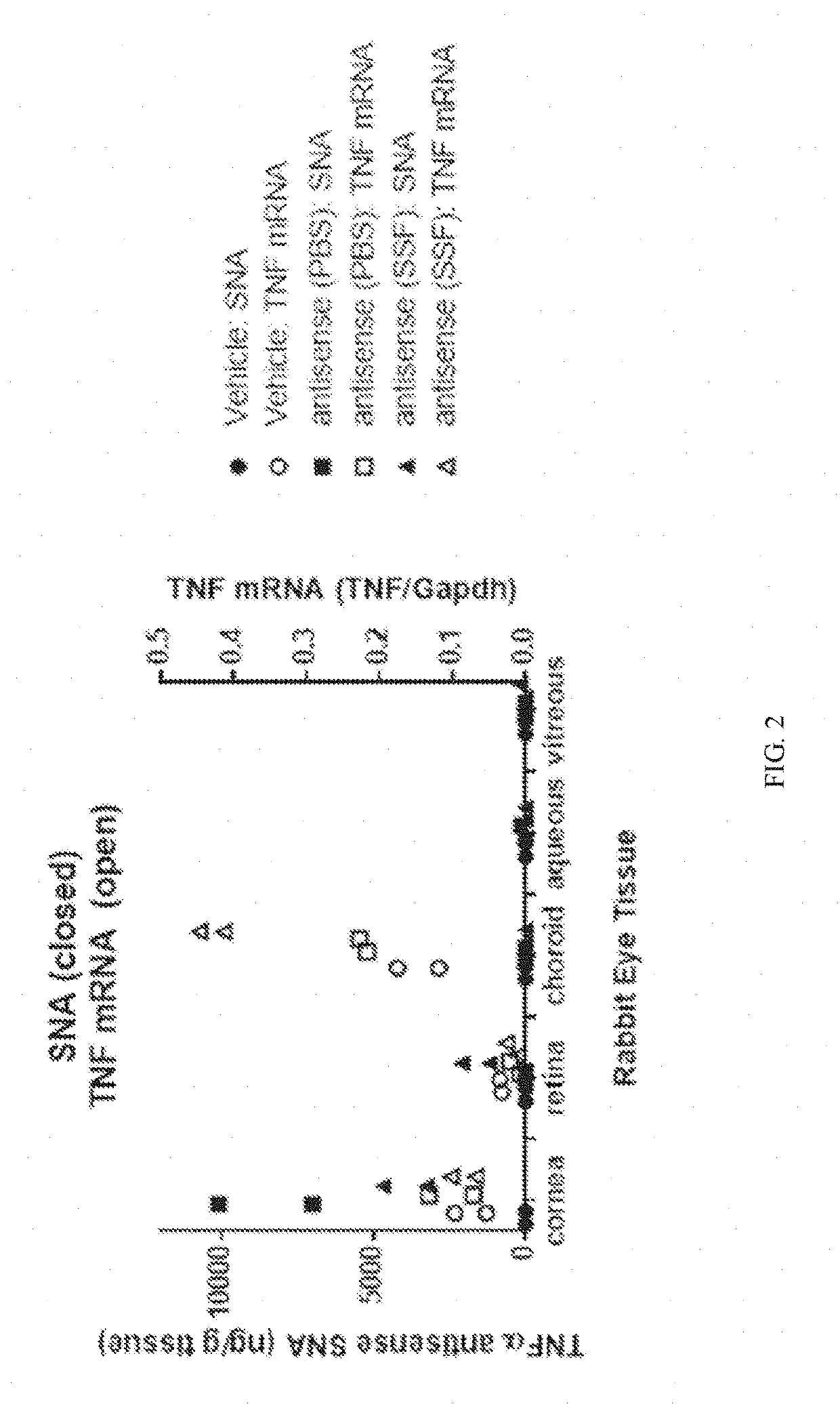
Aspects of the invention relate to topical and ocular formulations of spherical nucleic acids (SNA), as well as methods of use thereof and compositions thereof. The formulations may include an inhibitor such as an inhibitor of tumour necrosis factor alpha (TNFa), platelet-derived growth factor subunit A (PDGFA), platelet-derived growth factor subunit B (PDGFB), platelet-derived growth factor subunit C (PDGFC), platelet-derived growth factor subunit D (PDGFD), platelet-derived growth factor receptor alpha (PDGFRA), platelet-derived growth factor receptor beta (PDGFRB), platelet-derived growth factor receptor like (PDGFRL), vascular endothelial growth factor A (VEGFA), vascular endothelial growth factor B (VEGFB), vascular endothelial growth factor C (VEGFC) vascular endothelial growth factor D (VEGFD), vascular endothelial growth factor receptor-1 (VEGFR1), vascular endothelial growth factor receptor-2 (VEGFR2), vascular endothelial growth factor receptor-3 (VEGFR3), beta-2 adrenergic receptor (ADRB2), connective tissue growth factor (CTGF), interleukin 1 beta (IL1 β), interleukin 1 receptor-1 (IL1 R1), interleukin 1 receptor-2 (IL1R2), and interleukin 1 receptor-3 (IL1R3). Aspects of the invention further relate to nanostructures comprising self-assembling therapeutic oligonucleotides, such as antisense oligonucleotides, that are linked to a molecular species, wherein the molecular species is positioned in a core of the nanostructure and the oligonucleotides extend radially from the core.
Owner:EXICURE INC
HPV (human papillomavirus) peptide/DC (dendritic cell) mixed vaccine and preparation thereof
InactiveCN102008721APromote maturityImprove the level ofViral antigen ingredientsAntiviralsAdjuvantHuman papillomavirus
The invention provides an HPV (human papillomavirus) peptide / DC (dendritic cell) mixed vaccine prepared in the presence of a TLR (toll-like receptor) agonist. The mixed vaccine comprises one part of HPV11E77-15, one part of mouse bone marrow-derived dendritic cells which are cultured in vitro and one part of TLR9 agonist as an adjuvant, wherein the dendritic cells are extracted from mouse bone marrow and further cultured in vitro, the co-incubation with one section of HPV11E7CTL (cytotoxic T lymphocyte) epitope peptide with strongest immunogenicity is firstly carried out, the co-incubation with the TLR ligand CpG and the like is further respectively carried out for promoting the maturation of the DC, the degree of maturation can be confirmed by detecting the change of a marker on the surface of the DC after incubation, and the DC vaccine which is simulated to mature can be used for immunizing mice. The TLR ligand and the HPV11E7 peptide are utilized jointly to promote the degree of maturation of the DC, the level of TNF-alpha (tumor necrosis factor-alpha) and IFN-gamma (immunoreactive fibronectin-gamma) of factors of secretory cells of T cells can be particularly improved after combination of the CpG, and the HPV peptide / DC mixed vaccine can be used for preventing and treating genital warts and cervical cancer.
Owner:ZHEJIANG UNIV
Cancer Cell Apoptosis
InactiveUS20120190735A1Reduce proliferationLow toxicityBiocideNervous disorderB-cell apoptosisTumor necrosis factor alpha
There is described a therapeutic agent capable of directly or indirectly having an effect on the proteins N-methyl-D-aspartate (NMDA), Cyclooxygenase-2 (COX-2), Tumour Necrosis factor alpha (TNF-a), Nuclear factor-kappa B (NFKB), Cyclin-dependent kinases, e.g. CDK2 / A and CDK5 / p25, Histone acetyltransferase (HAT) and Farnesyltransferase, simultaneously, sequentially or separately. There is especially described dexanabinol, or a derivative thereof, as the therapeutic agent.
Owner:E THERAPEUTICS LTD
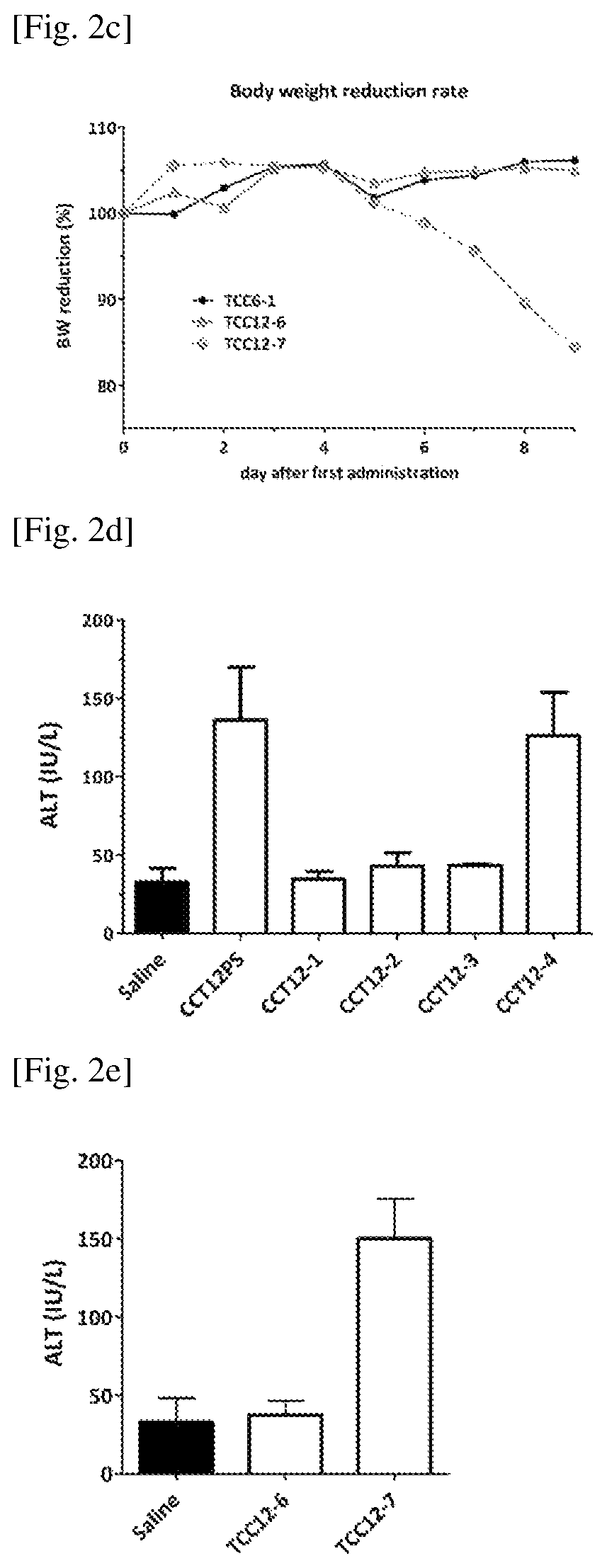
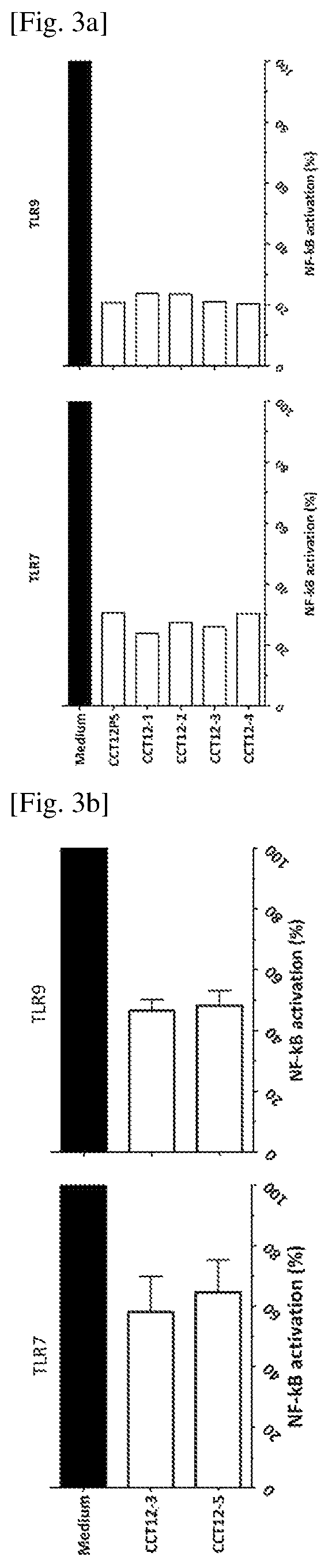
Inhibitory Oligonucleotide and Use Thereof
ActiveUS20150299710A1Inhibiting cytokine productionInhibit productionAntibacterial agentsOrganic active ingredientsNf κb activationTLR9
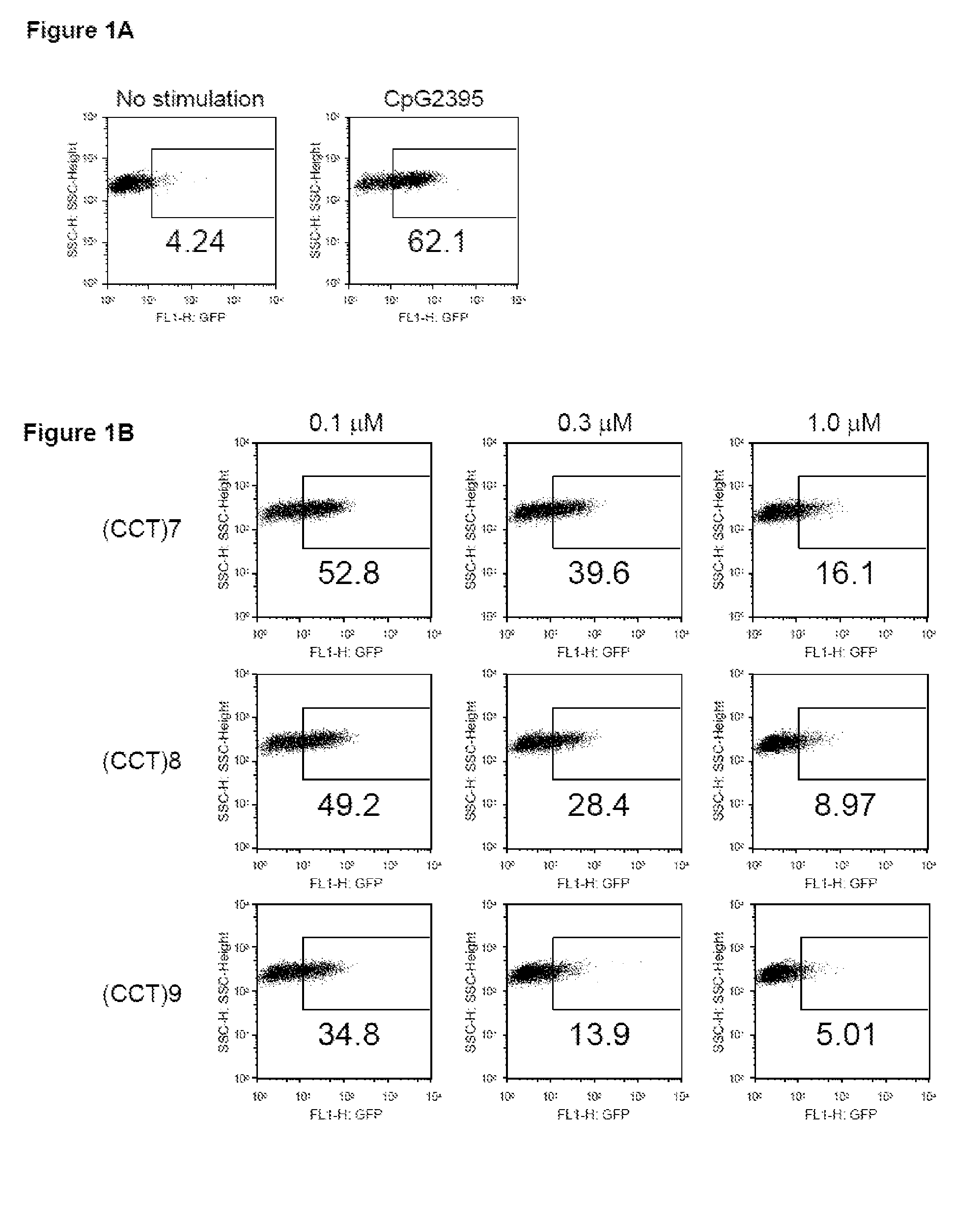
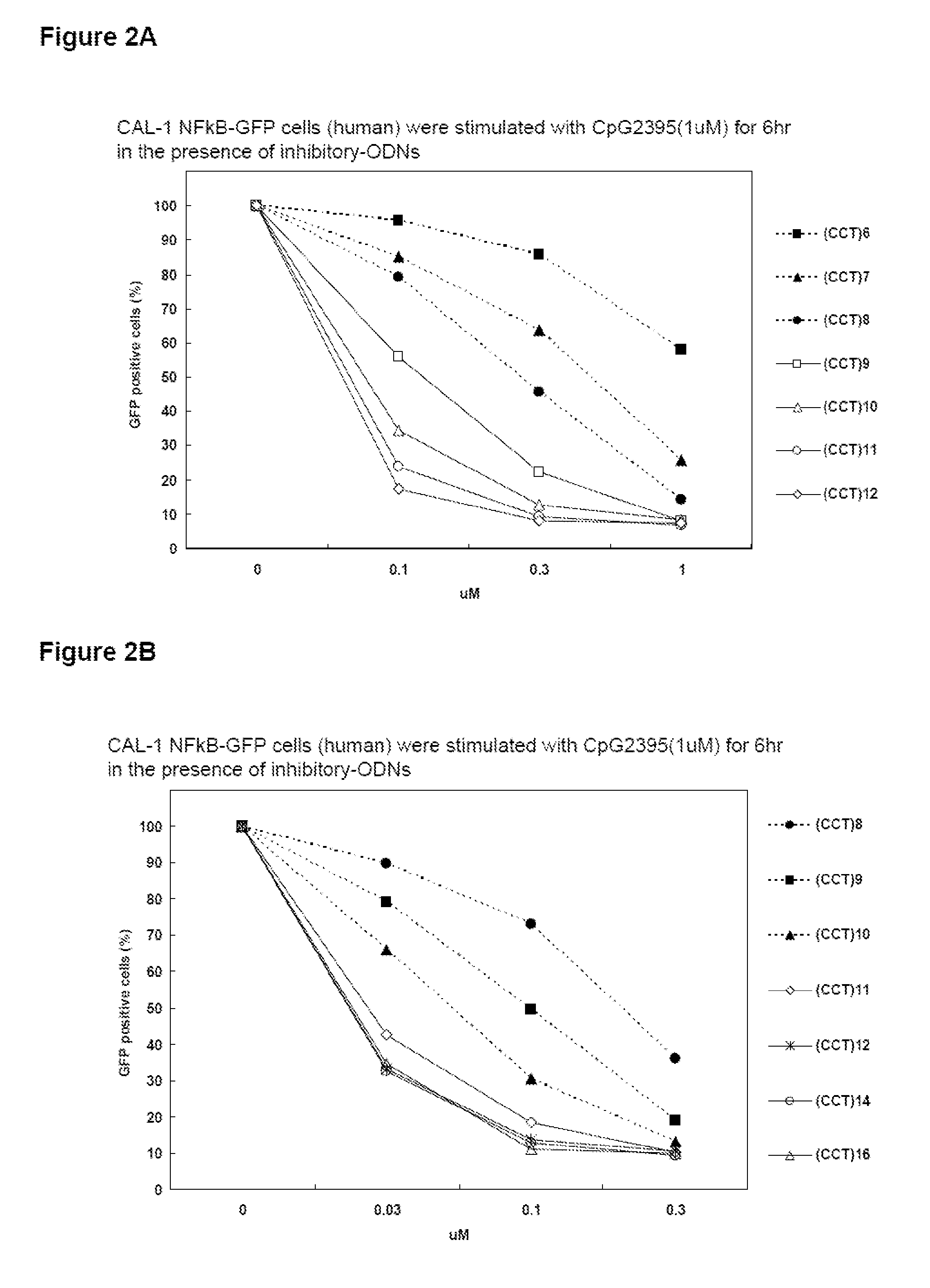
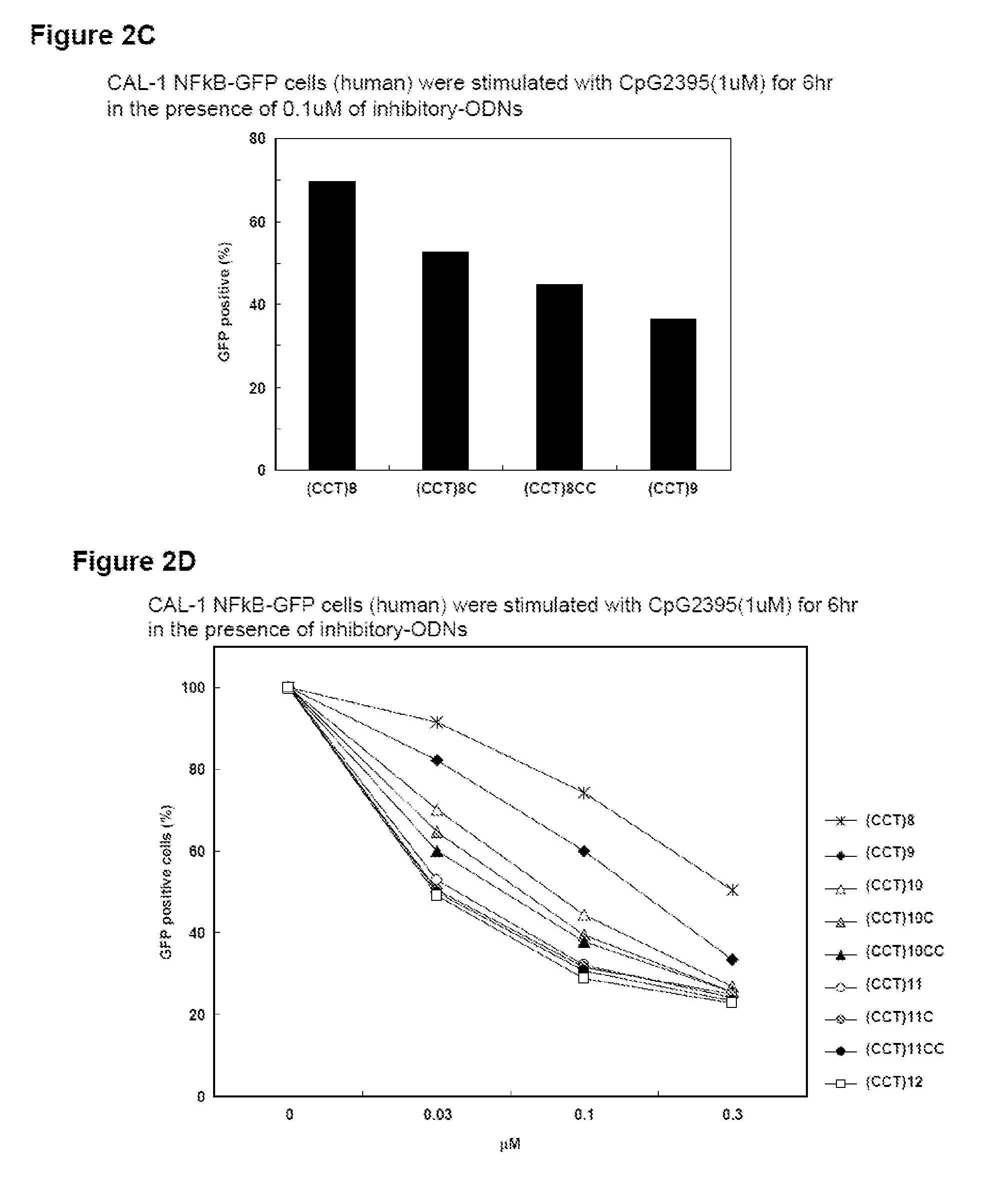
The inhibitory oligonucleotides (ODNs) which strongly block NF-κB activation induced by TLR9 agonists and TLR7 agonists are provided. The production of proinflammatory cytokines, such as interleukin-6 and tumor necrosis factor alpha, is inhibited by the inhibitory-ODNs. Interferon production from human PBMC induced by TLR9 agonist is prevented by the inhibitory-ODNs. These ODNs can be used as a remedy for the treatment of immune-mediated disorders such as rheumatoid arthritis, systemic lupus erythematosus (SLE), sepsis, multiple organ dysfunction syndromes.
Owner:SBI BIOTECH CO LTD
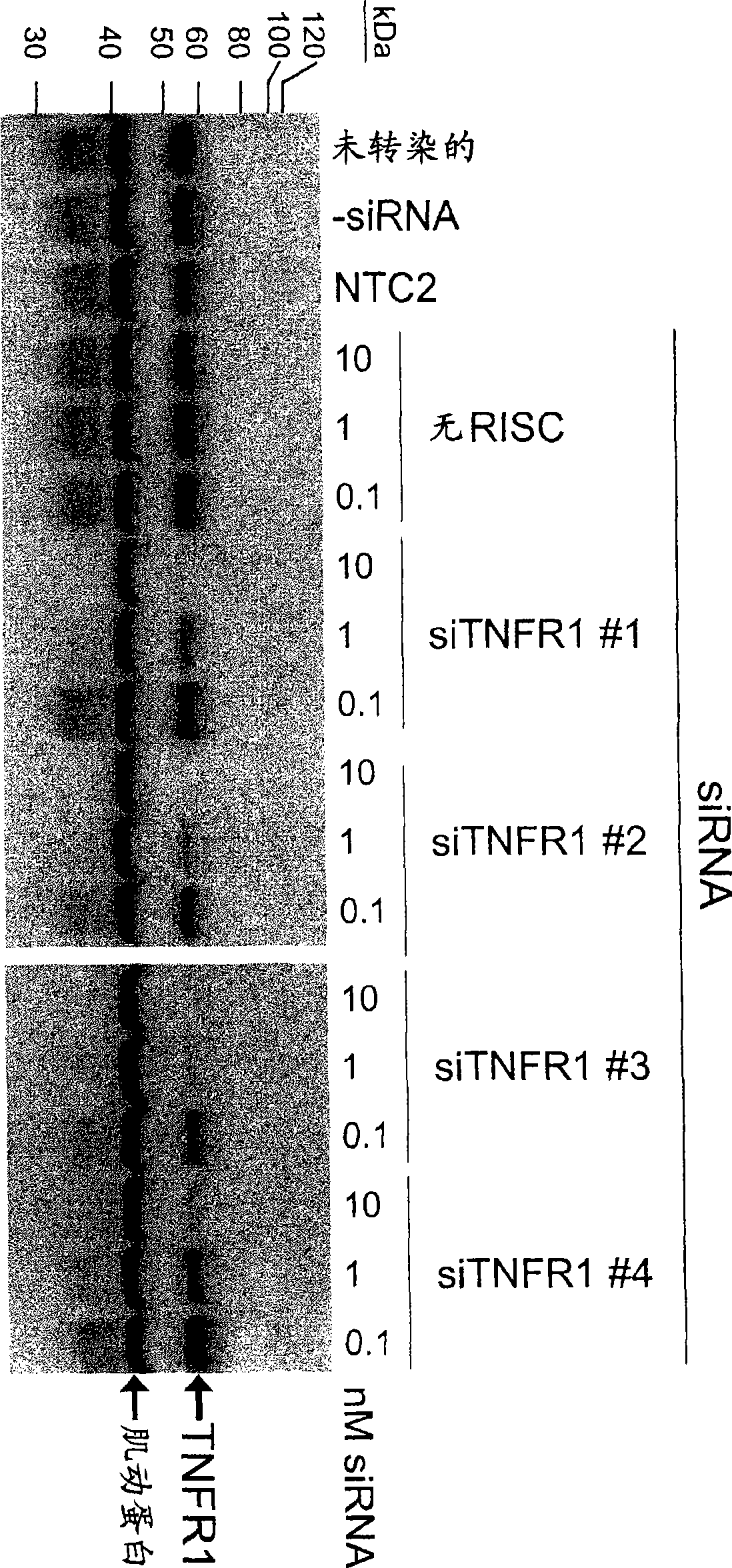
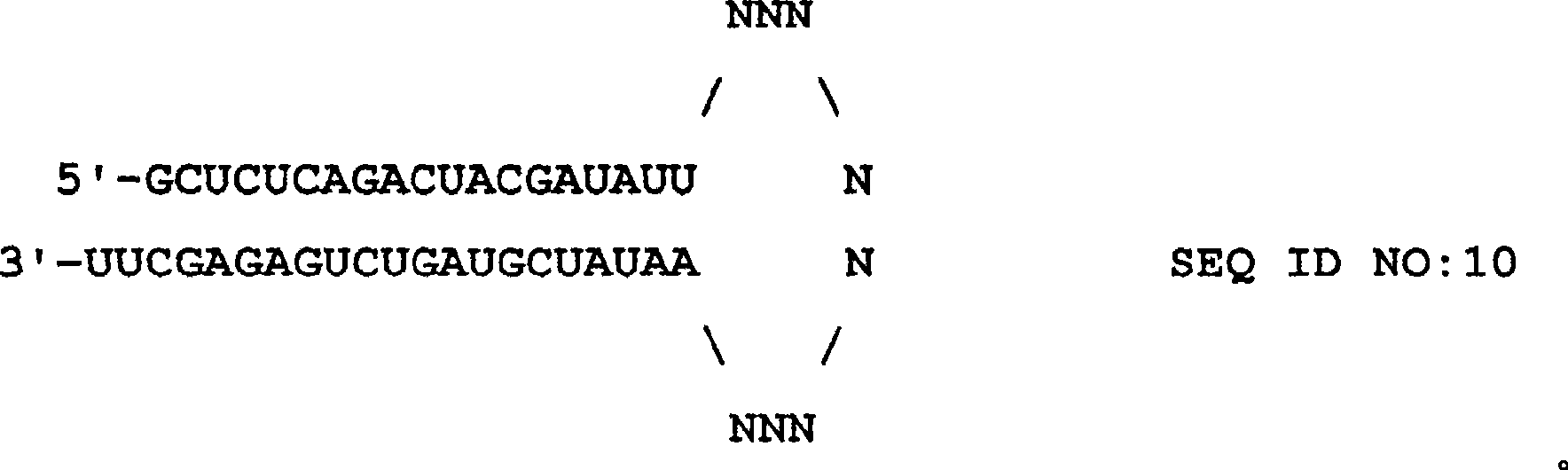
RNAi-mediated inhibition of tumor necrosis factor alpha-related conditions
The present invention provides a RNA interferencefor inhibition of tumor necrosis factor a (TNFa) by silencing TNFa cell surface receptor TNF receptor-1 (TNFR1) mRNA expression, or by silencing TNFa converting enzyme (TACE / ADAM17) mRNA expression. Silencing such TNFa targets, in particular, is useful for treating patients having a TNFa-related condition or at risk of developing a TNFa-related condition such as the ocular conditions dry eye, allergic conjunctivitis, or ocular inflammation, or such as dermatitis, rhinitis, or asthma, for example.
Owner:ARROWHEAD RES CORP
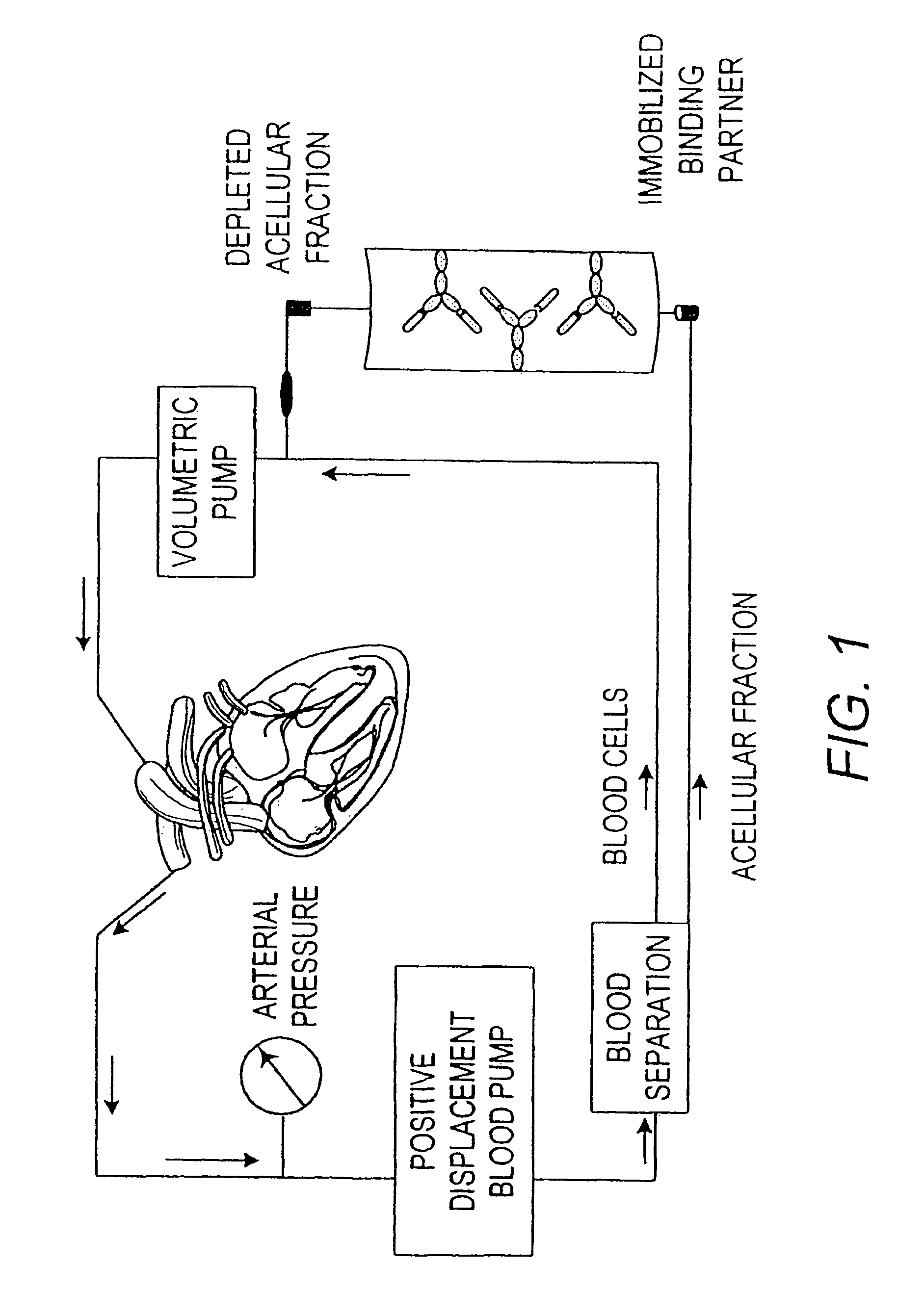
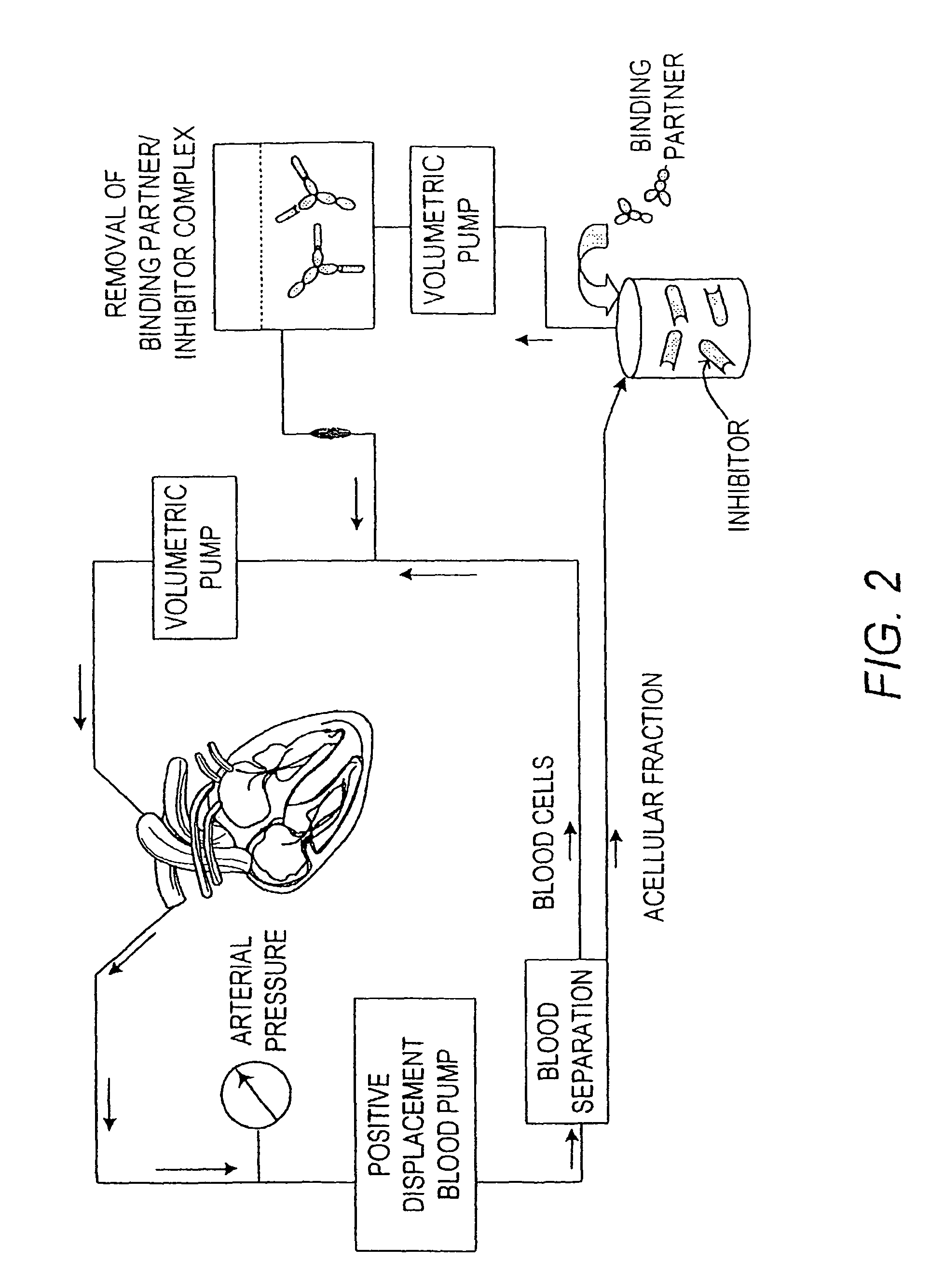
Immobilized tumor necrosis factor-alpha muteins for enhancing immune response in mammals
ActiveUS8501918B2Reduce abundanceMinimize ToxicityAntibacterial agentsAntimycoticsMammalTumour necrosis factor alpha
The present invention provides a method for enhancing an immune response in a mammal to facilitate the elimination of a chronic pathology. The method involves the removal of immune system inhibitors such as soluble TNF receptor from the circulation of the mammal, thus, enabling a more vigorous immune response to the pathogenic agent. The removal of immune system inhibitors is accomplished by contacting biological fluids of a mammal with one or more binding partner(s) such as TNFα muteins capable of binding to and, thus, depleting the targeted immune system inhibitor(s) from the biological fluids. Particularly useful in the invention is an absorbent matrix composed of an inert, biocompatible substrate joined covalently to a binding partner, such as a TNFα mutein, capable of specifically binding to a targeted immune system inhibitor such as soluble TNF receptor.
Owner:THOMPSON ONCOLOGY DEVICES LLC
Electrochemiluminescence detection method for tumor necrosis factor alpha and kit of electrochemiluminescence detection method
InactiveCN109164090AHigh detection sensitivityAvoid interferenceChemiluminescene/bioluminescenceMaterial electrochemical variablesQuantum yieldSmall sample
The invention discloses an electrochemiluminescence detection method for a tumor necrosis factor alpha and a kit of the electrochemiluminescence detection method. According to the method, the tumor necrosis factor alpha is adopted as a detection target, a high quantum yield gold nano cluster electrochemiluminescence technique and an immunoassay technique are organically combined, a high quantum yield gold nano cluster electrochemiluminescence probe is prepared by using a reduction method, a manganese dioxide nano material is adopted as an electrochemiluminescence quenching agent, an electrochemiluminescence signal is recovered through a redox reaction between ascorbic acid generated from enzyme-linked immunosorbent assay and manganese dioxide, and the method is a high-performance electrochemiluminescence tumor necrosis factor alpha detection method based on the high quantum yield gold nano cluster probe. The method has a linear range of 0.06-31pg / mL for tumor necrosis factor alpha detection, and has a detection limit of 36fg / mL. The method has the characteristics of rapidness, accuracy, high sensitivity, good selectivity, good stability, small sample use amount and the like, and has relatively good clinical application prospects.
Owner:FUJIAN MEDICAL UNIV
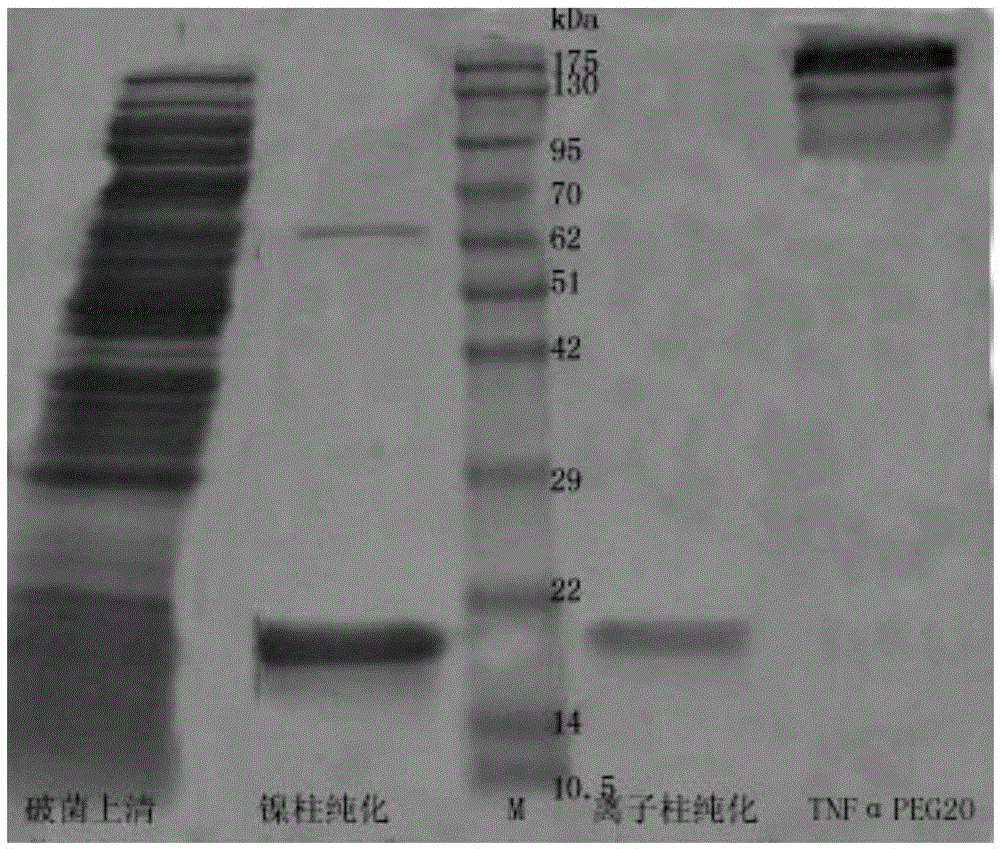
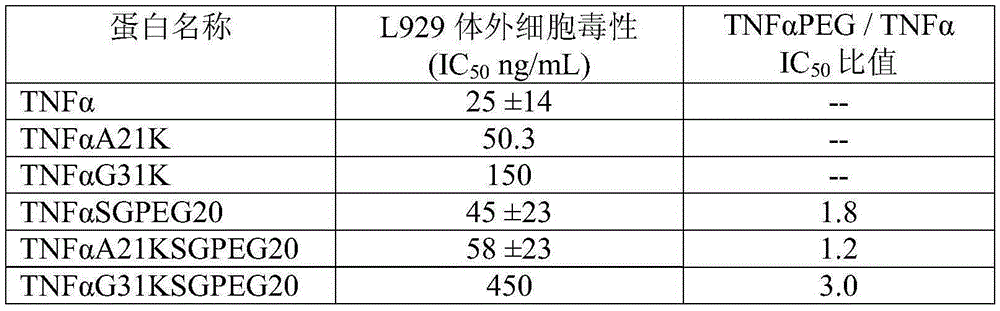
Conjugate of polyethylene glycol and tumor necrosis factor alpha or analogue of polyethylene glycol and tumor necrosis factor alpha and medical application of conjugate
The invention relates to conjugate of polyethylene glycol and a tumor necrosis factor alpha or analogue of the polyethylene glycol and the tumor necrosis factor alpha and medical application of the conjugate, in particular to conjugate of polyethylene glycol-tumor necrosis factor alpha or analogue of the polyethylene glycol-tumor necrosis factor alpha, a preparation method of the conjugate and application of the conjugate serving as a therapeutic agent in preventing or treating tumor or cancer. The conjugate of polyethylene glycol-tumor necrosis factor alpha or analogue of the polyethylene glycol-tumor necrosis factor alpha can lower acute toxicity of medicine, reduce the intravenous dosing frequency, reduce the dosage of applied medicine, increase the anti-tumor therapeutic effect, solve the problem that efficacy half-life of the tumor necrosis factor alpha protein is short through parenteral medicine application, achieve the target of preventing and treating tumor or cancer and thus has beneficial medical prospect.
Owner:岳阳新华达制药有限公司
Protein ACA1 of antrodia camphorata
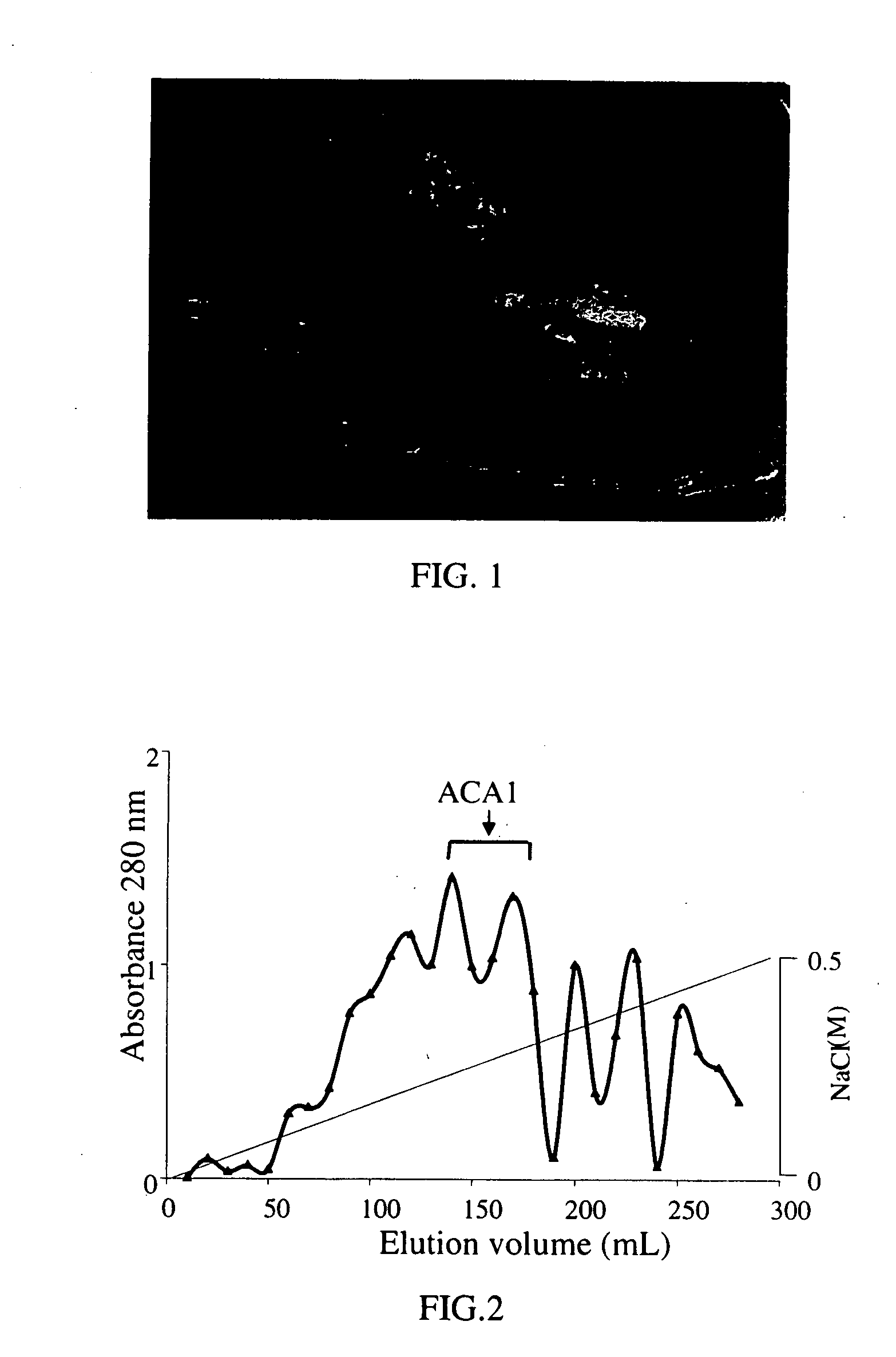
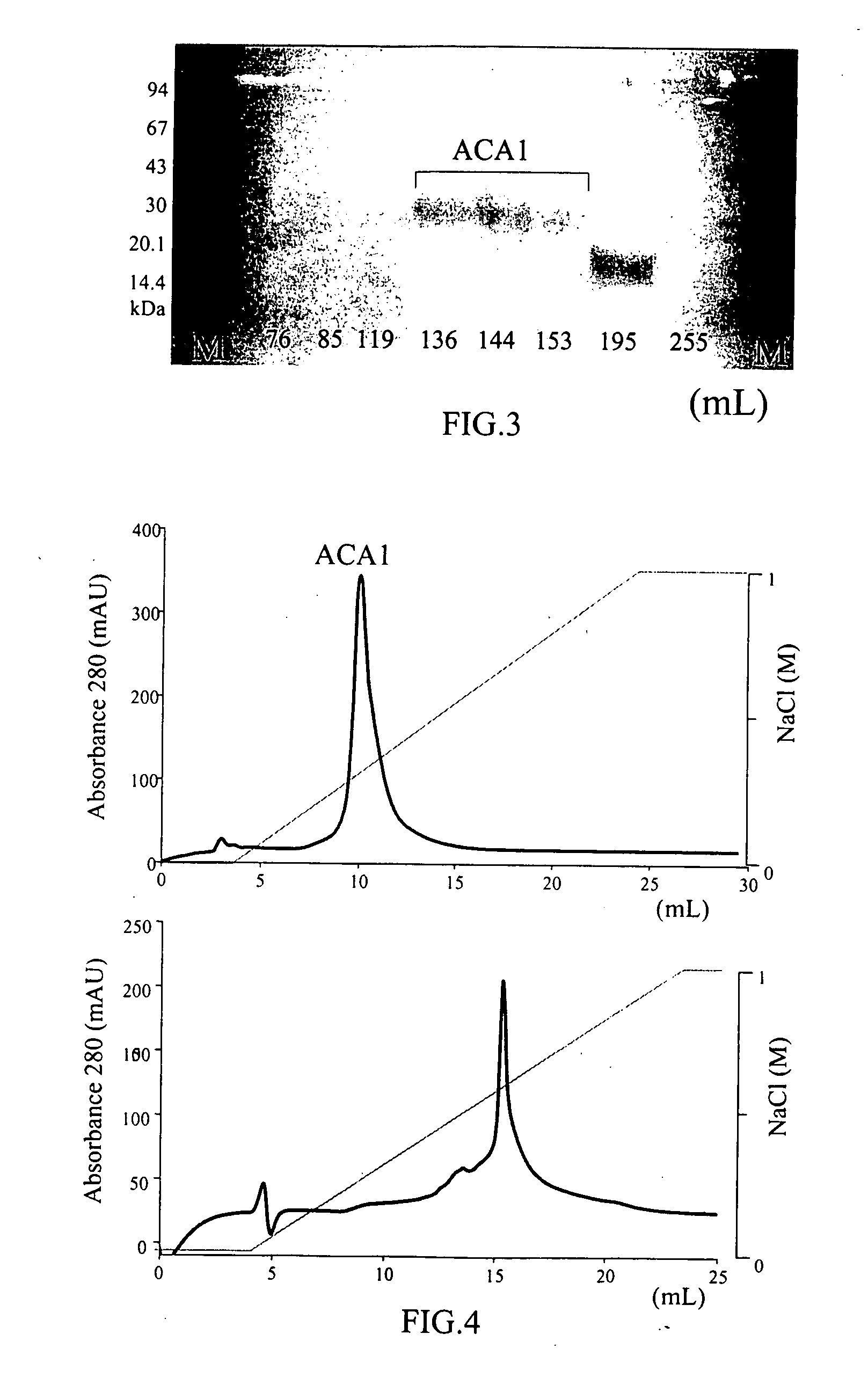
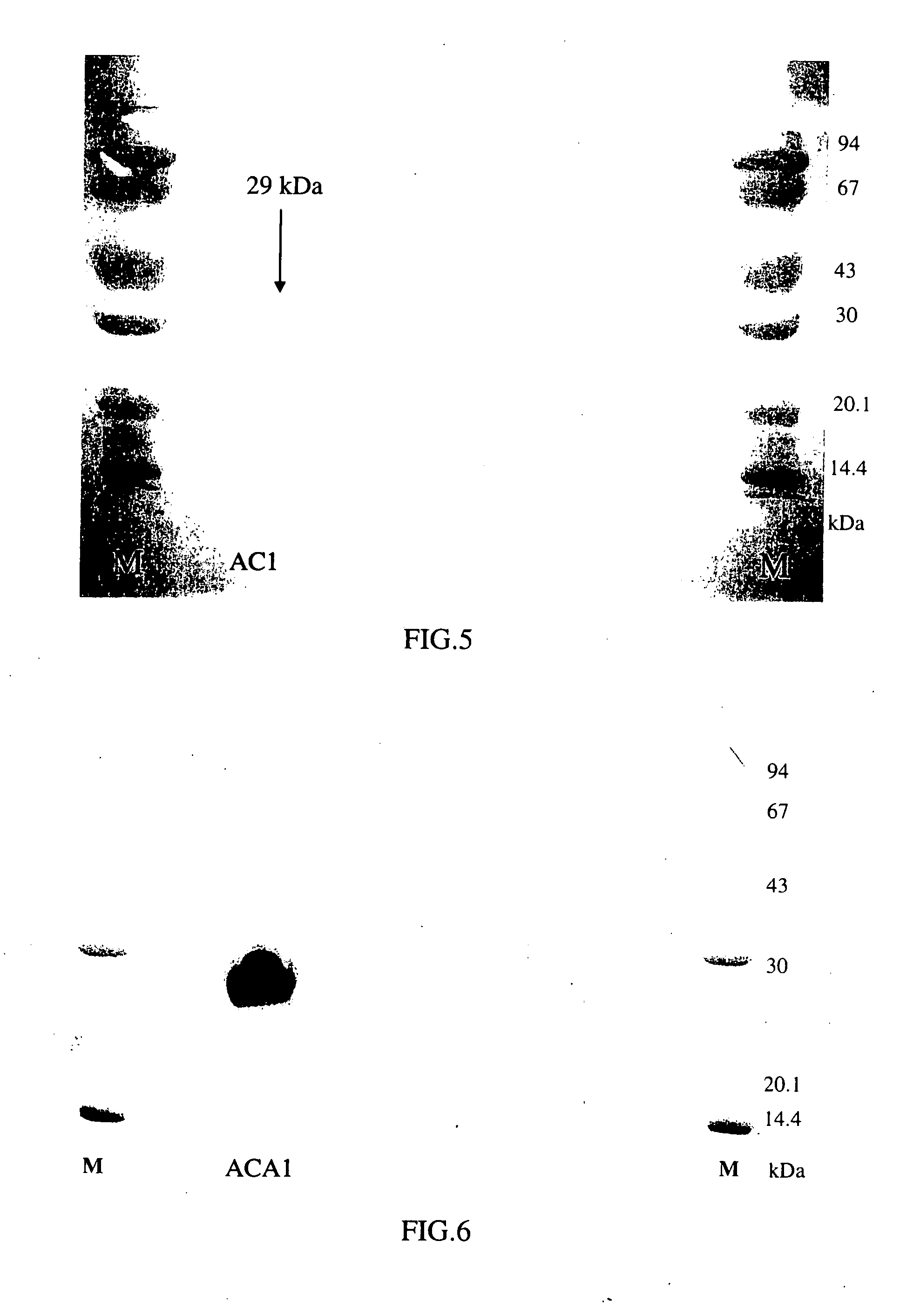
InactiveUS20050164931A1Increase productionInduce cell proliferationFungiSugar derivativesFungal microorganismsHalf-Cystine
A new protein, named ACA1, has been isolated and purified from the medical fungi Antrodia camphorata using the technique of anion-exchange chromatography. ACA1, a glycoprotein with a molecular mass of 29 kDa, has a pI value of pH 5.3 and contains 118 amino acids in its peptide moiety. In addition, ACA1 contains methionine, half-cystine and histidine residues, which are not existent in FIP-fve and Ling Zhi-8. ACA1 is not able to agglutinate red blood cells from human and mouse. Moreover, ACA1 possesses immunomodulatory activities, which are demonstrated by their stimulatory activity toward RAW 264.7 macrophages and mouse splenocytes. ACA1 can directly enhance the production of tumor necrosis factor-alpha and nitric oxide by RAW 264.7 macrophages, and induce cell proliferation and interferon-gamma secretion by mouse splenocytes.
Owner:CHIEN PO JUNG
Topical treatment of immune checkpoint inhibitor induced diarrhoea, colitis or enterocolitis using antibodies and fragments thereof
PendingCN113166238ASlow or relieve diarrheaPowder deliveryDigestive systemEnterocolitisTopical treatment
The present invention relates to the therapeutic topical use of compositions containing antibody molecules or functional fragments or derivatives specific to tumour necrosis factor alpha (TNF[alpha]), for treating or preventing immune checkpoint (ICP) inhibitor-induced adverse events.
Owner:蒂洛特斯制药股份有限公司
Zone 2 protein of programmed cell death protein 2 analogue and uses thereof
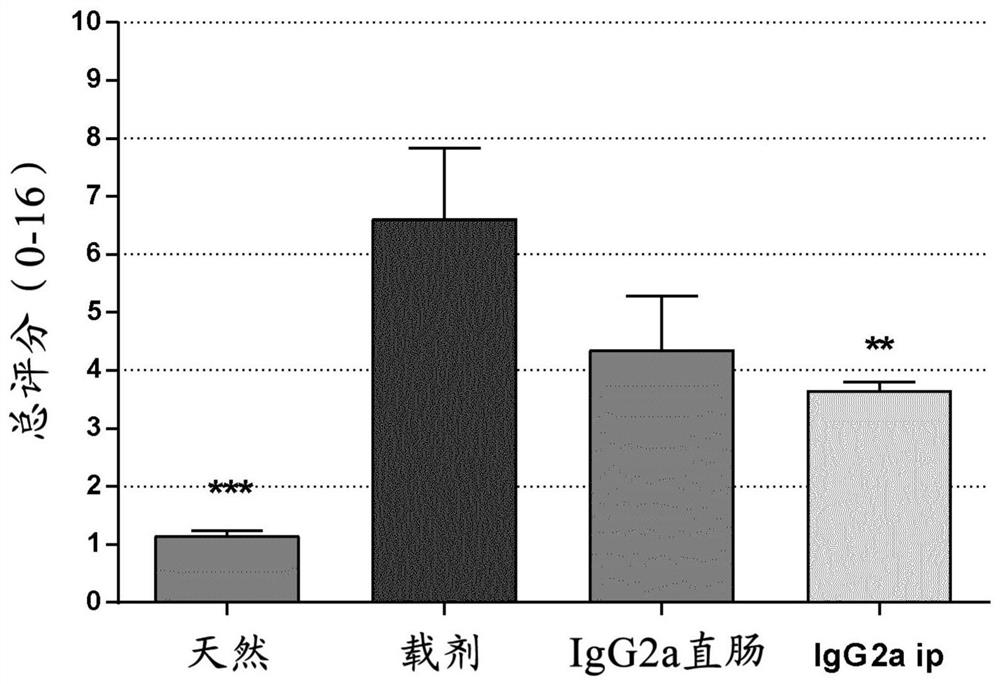
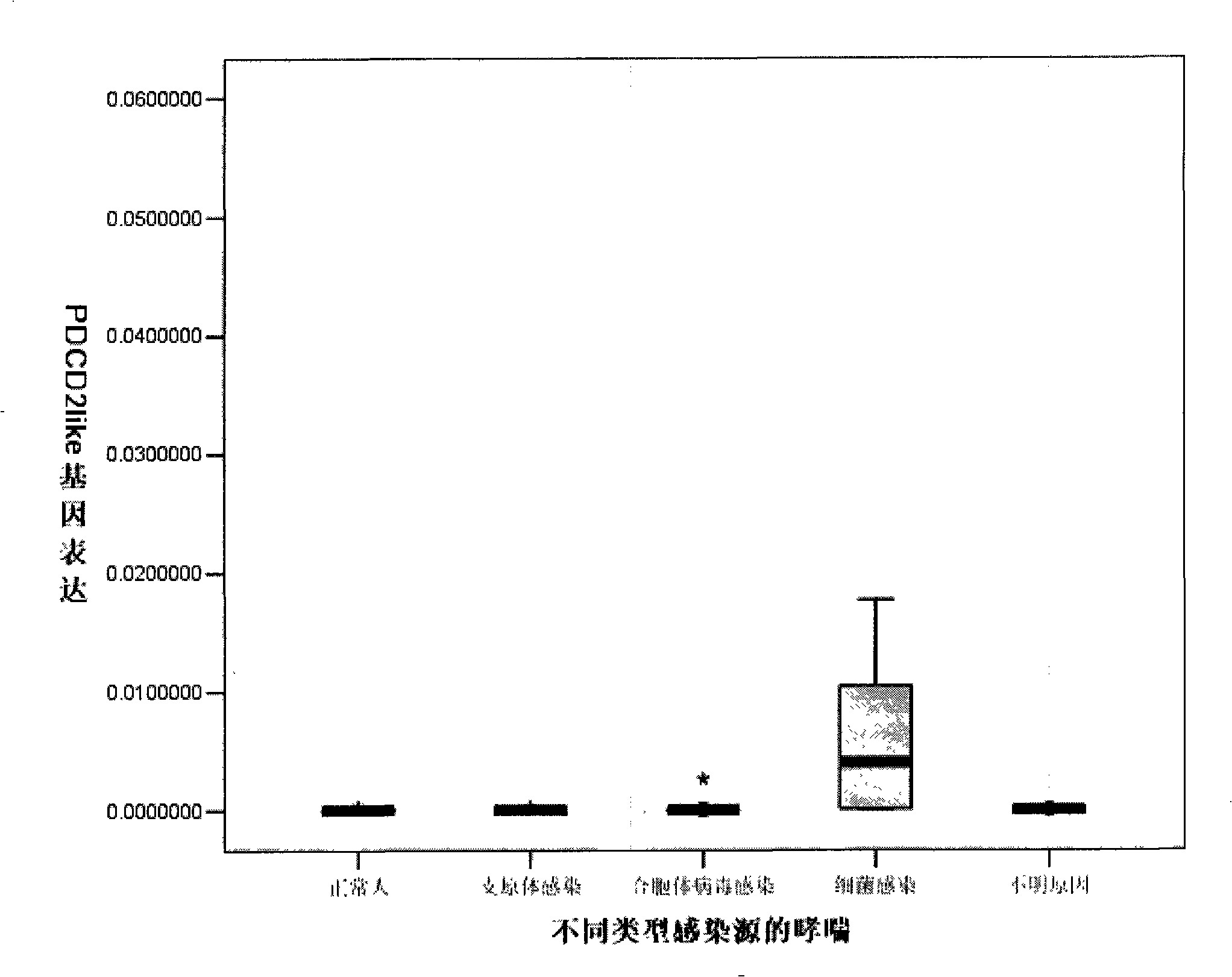
InactiveCN101353378AAddressing drug resistanceInhibitionAntibacterial agentsOrganic active ingredientsFactor iiTumor necrosis factor alpha
The invention discloses a protein segment of programmed death protein 2 analog, namely, the protein of region 2, and the application of the protein of the region 2 in preparing drugs for curing bacterial inflammation of the programmed death protein 2 analog. The protein of region 2 of the programmed death protein 2 analog realizes the function of restraining the tumor necrosis factor Alpha of a pro-inflammatory cytokine caused by bacterial LPS, thereby restraining the generating and developing of the inflammations caused by bacteria.
Owner:SUZHOU UNIV
Tlr inhibitory oligonucleotides and their use
PendingCN108431228AInhibitory activity does not significantly affectOrganic active ingredientsActivity regulationDiseaseWhite blood cell
The inhibitory oligonucleotides with partial phosphorothioation with reduced toxicity strongly block NF-kB activation induced by TLR9 agonists and TLR7 / 8 agonists. The production of proinflammatory cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor alpha (TNFa), is inhibited by the inhibitory-oligonucleotides. Interferon (IFN) production from human PBMC induced by TLR9 agonist is prevented by the inhibitory-oligonucleotides. These oligonucleotides can be used as a remedy for the treatment of immune-mediated disorders such as rheumatoid arthritis, systemic lupus erythematosus (SLE), sepsis, multiple organ dysfunction syndromes and inflammatory cytokine-mediated inflammatory disease.
Owner:SBI BIOTECH CO LTD
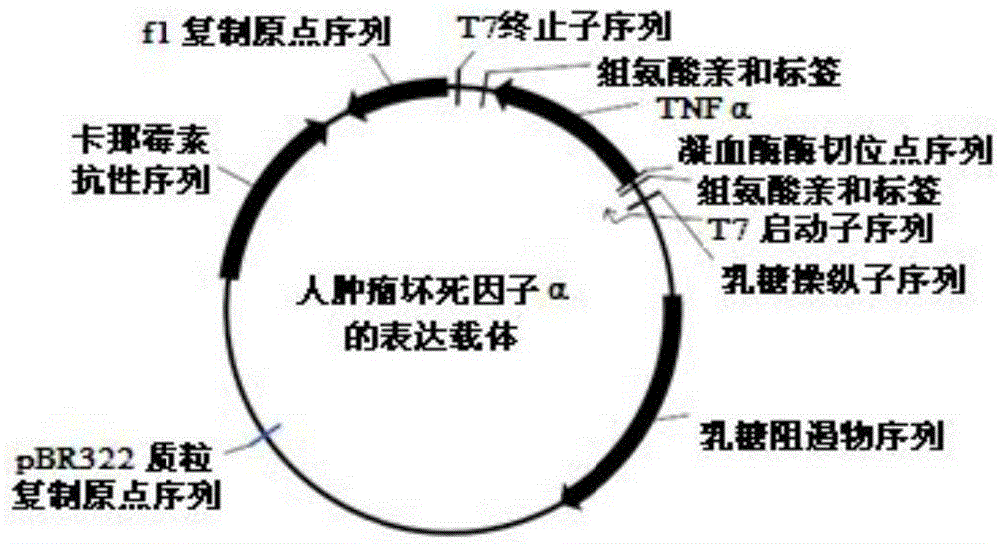
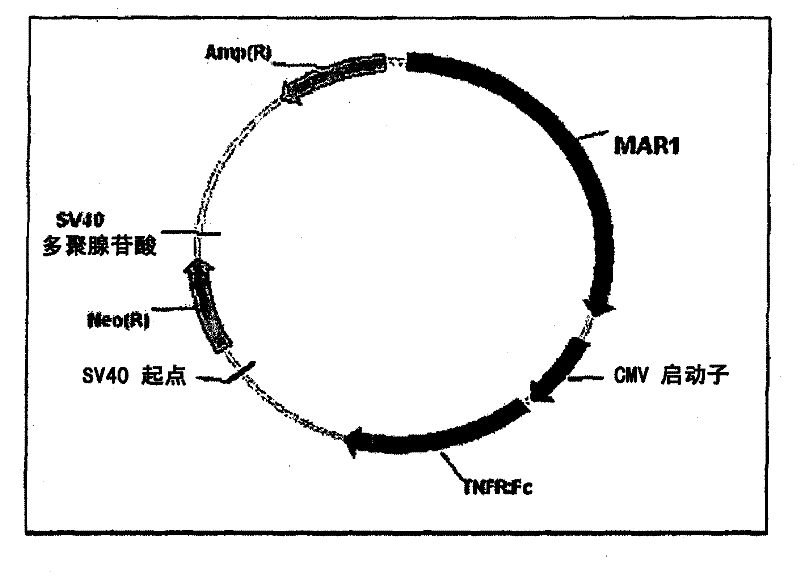
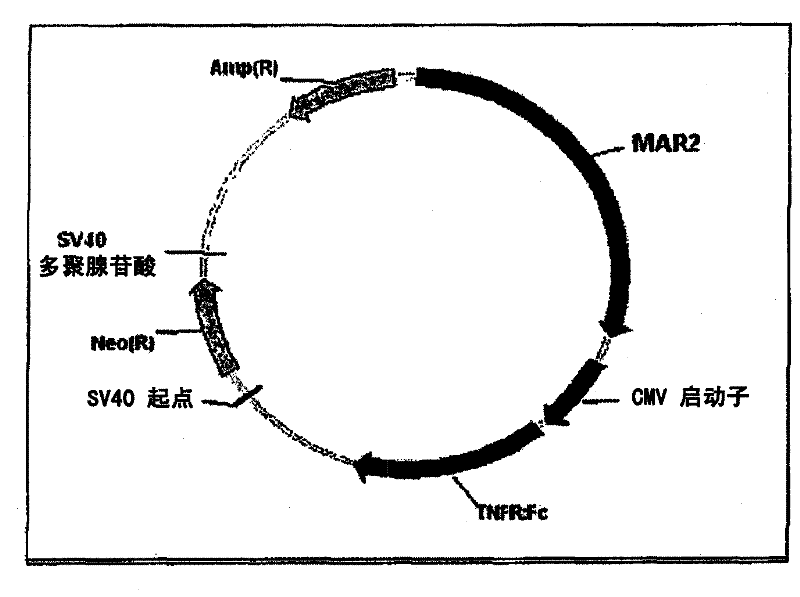
An expression vector and a method thereof
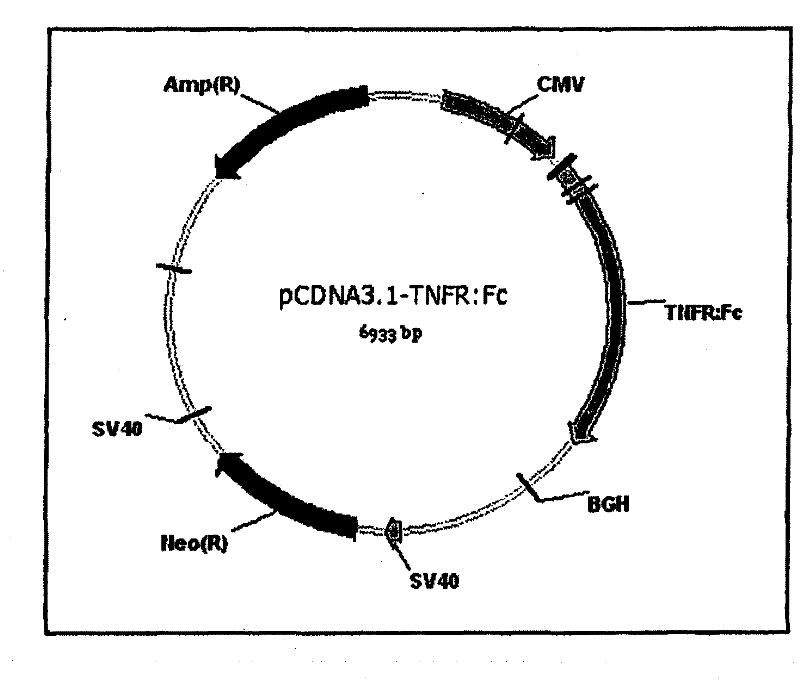
InactiveCN102177240AAntibody mimetics/scaffoldsReceptors for cytokines/lymphoines/interferonsTumour necrosis factor alphaReceptor for activated C kinase 1
The present invention relates to vectors and compounds for the expression of recombinant soluble proteins. More particularly, the present invention relates to nucleic acid molecules, expression vectors, and host cells for the expression of recombinant soluble Tumour Necrosis Factor Alpha receptor (TNFR) - Human IgG Fc fusion protein. The invention further relates to methods for preparing recombinant soluble Tumour Necrosis Factor Alpha receptor (TNFR) - Human IgG Fc fusion protein using the host cells transfected with the expression vectors.
Owner:AVESTHAGEN
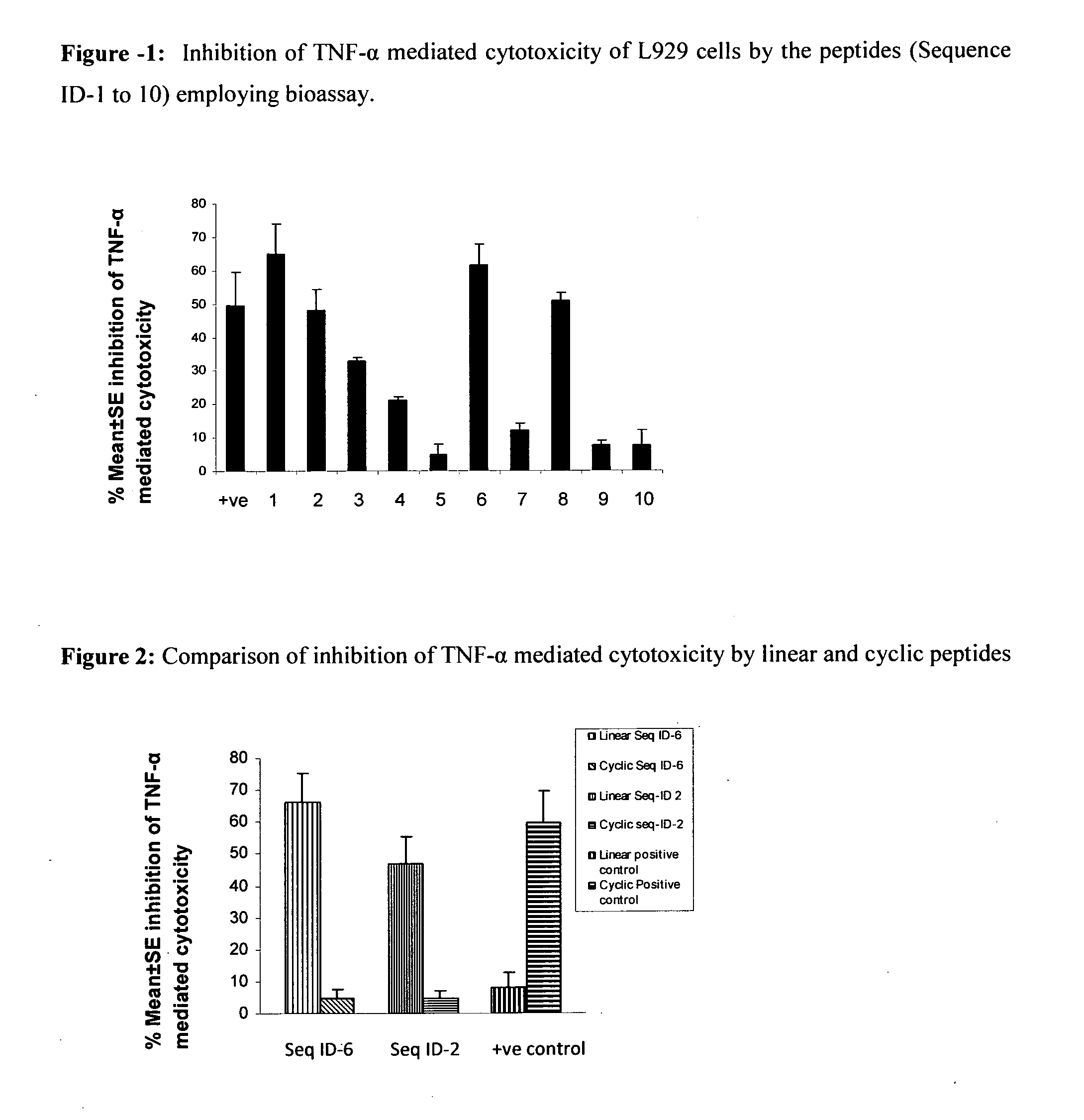
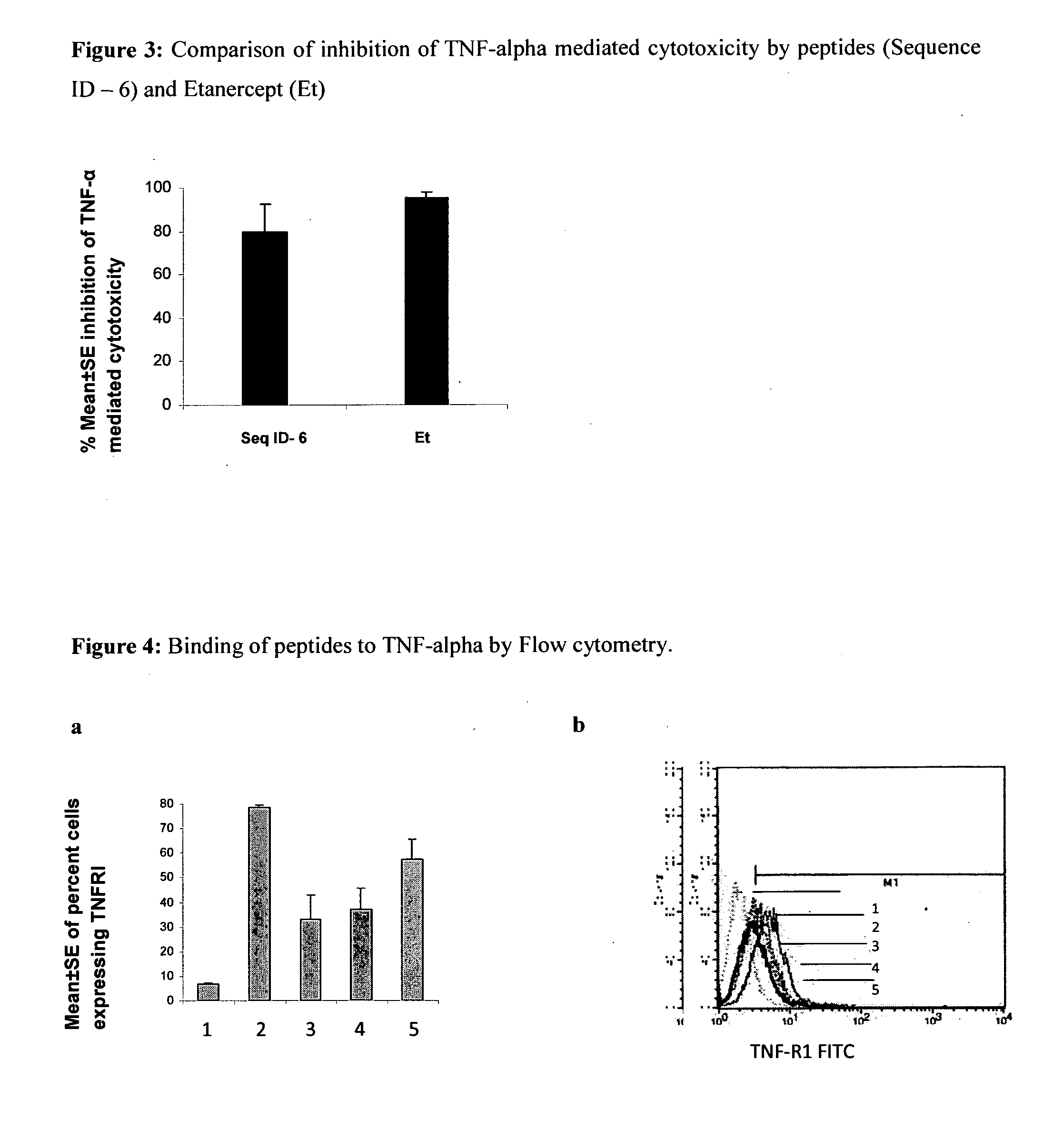
Tumor necrosis factor inhibiting peptides and uses thereof
The present invention relates to Tumor Necrosis Factor-alpha (TNF-alpha or TNF-α) inhibiting peptides and process for the preparation thereof. The present invention further relates to a pharmaceutical composition comprising TNF-alpha inhibiting peptides of the present invention and uses thereof in treating TNF-alpha mediated inflammatory disorders.
Owner:PANACEA BIOTEC
1-or 3-thia-benznaphthoazulenes as inhibitors of tumour necrosis factor production and intermediates for the preparation thereof
InactiveUS20050137249A1Antibacterial agentsBiocideAntiinflammatory EffectTumour necrosis factor alpha
The present invention relates to 1- or 3-thiabenzonaphthoazulene derivafives to their pharmacologically acceptable salts and solvates, to processes and intermediates for the preparation thereof as well as to their antiinflammatory effects, especially to the inhibition of tumour necrosis factor-alpha (TNF-alpha) production and the inhibition of interleukin-1 (IL-1) production as well as to their analgetic action.
Owner:GLAXOSMITHKLINE ISTRAZIVACKI CENTAR ZAGREB D O O
Protein vaccine aiming at tumor necrosis factor alpha and applications of protein vaccine
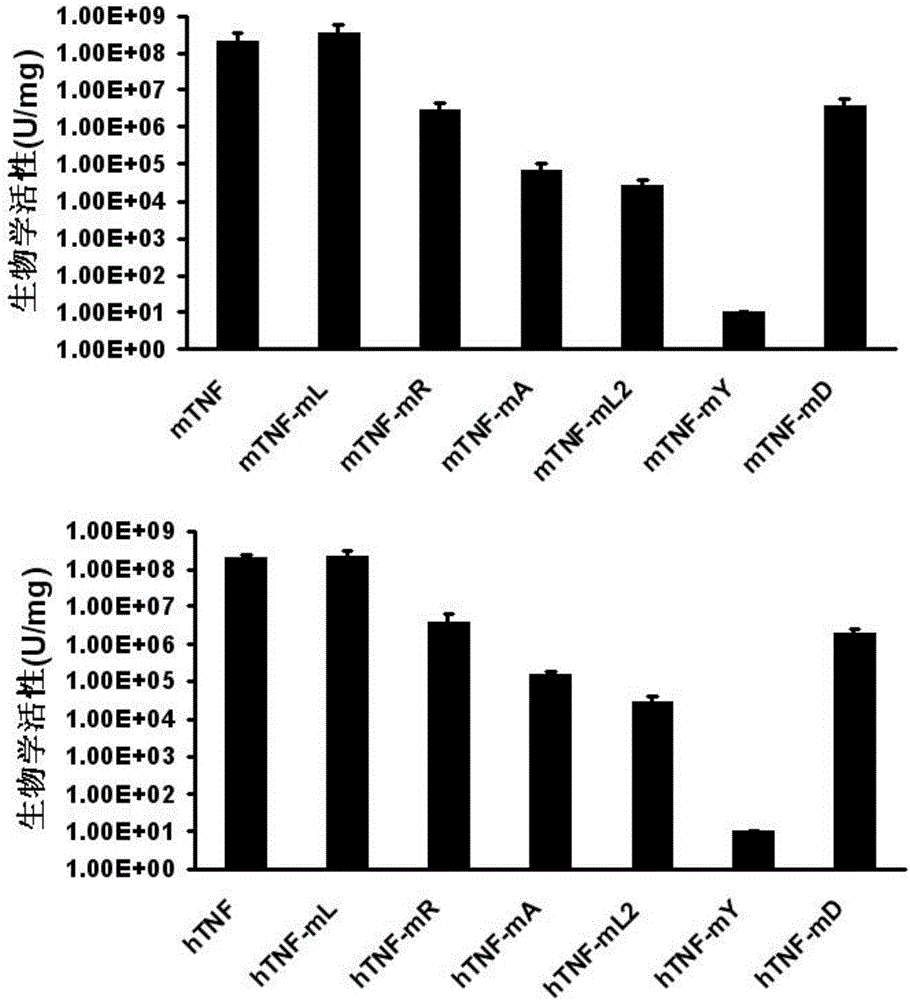
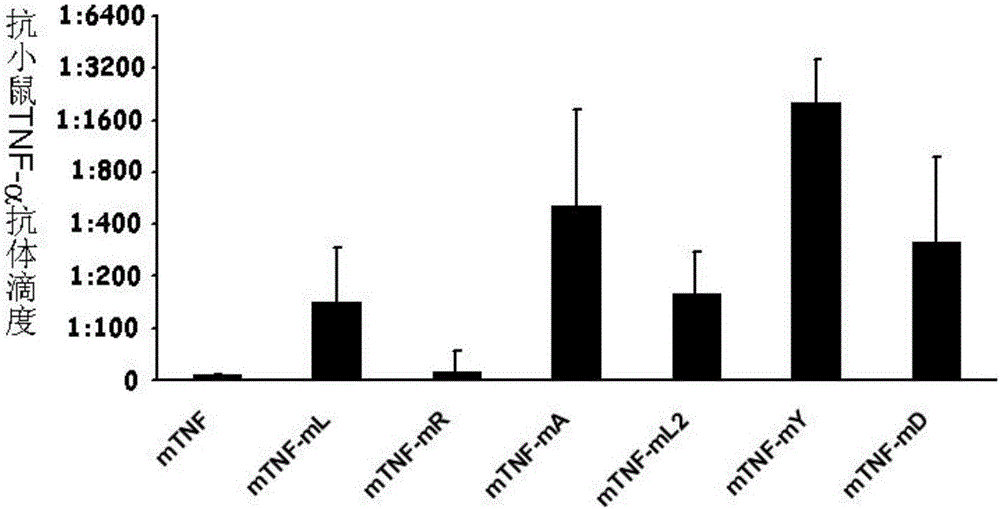
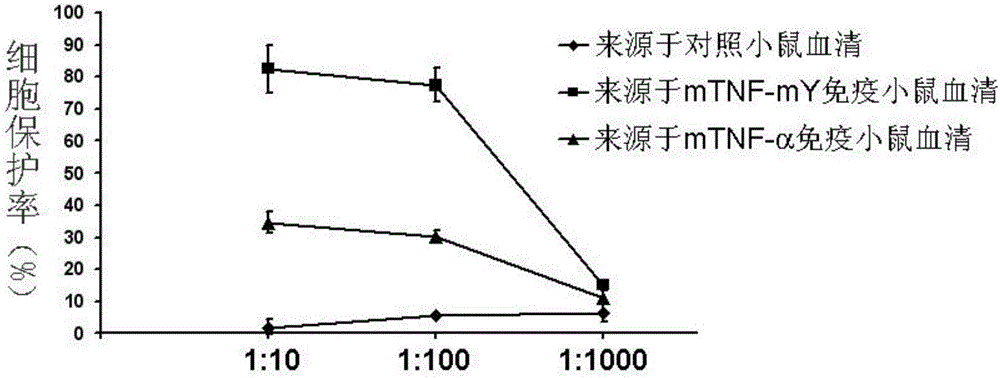
ActiveCN106540252AInhibit biological activityInhibit the inflammatory responseAntipyreticAntibody mimetics/scaffoldsImmunologic disordersMutated protein
The invention belongs to the field of biological medicines, and relates to a mutant protein vaccine aiming at tumor necrosis factor alpha (TNF-alpha) and applications of the mutant protein vaccine. The invention aims at providing the protein vaccine which is good in property and takes TNF-alpha as the target spot. According to the scheme, by immunizing the TNF-alpha mutant protein vaccine, the biological activity of the TNF-alpha mutant protein vaccine is inhibited, the cross immunologic reaction is generated in vivo, the biological functions of TNF-alpha are neutralized, and the autoimmune diseases and inflammatory diseases are inhibited. The protein vaccine aiming at TNF-alpha can be used for treating the autoimmune diseases and the inflammatory diseases and preventing the relapse of the autoimmune diseases and the inflammatory diseases.
Owner:SICHUAN UNIV
TLR inhibitory oligonucleotides and their use
ActiveUS10806749B2Increase doseSafer compositionOrganic active ingredientsActivity regulationDiseaseWhite blood cell
Owner:SBI BIOTECH CO LTD
Preparation method and application of effective component of artemisia sieversiana
The invention relates to a preparation method and an application of an effective component of artemisia sieversiana. The effective component is obtained as follows: a low-polarity component is obtained after artemisia sieversiana is subjected to conventional soaking extraction, heating reflux extraction, cold leaching extract, microwave assisted extraction or ultrasonic assisted extraction and removal of a solvent under reduced pressure, the low-polarity component is subjected to extraction with n-hexane after being dissolved and dispersed in water, an extraction part is dissolved in acetonitrile after being dried by distillation, filtrate is dried by distillation after filtration, and the effective component of the artemisia sieversiana is obtained. The effective component of the artemisia sieversiana, which is obtained with the method, proves in vitro that the effective component can be used for preventing and treating inflammation caused by excess of NO, TNF-alpha (tumor necrosis factor-alpha), IL-6 (interleukin-6) and MCP-1 (monocyte chemoattractant protein-1) and can be applied to anti-inflammatory drugs.
Owner:XINJIANG TECHN INST OF PHYSICS & CHEM CHINESE ACAD OF SCI
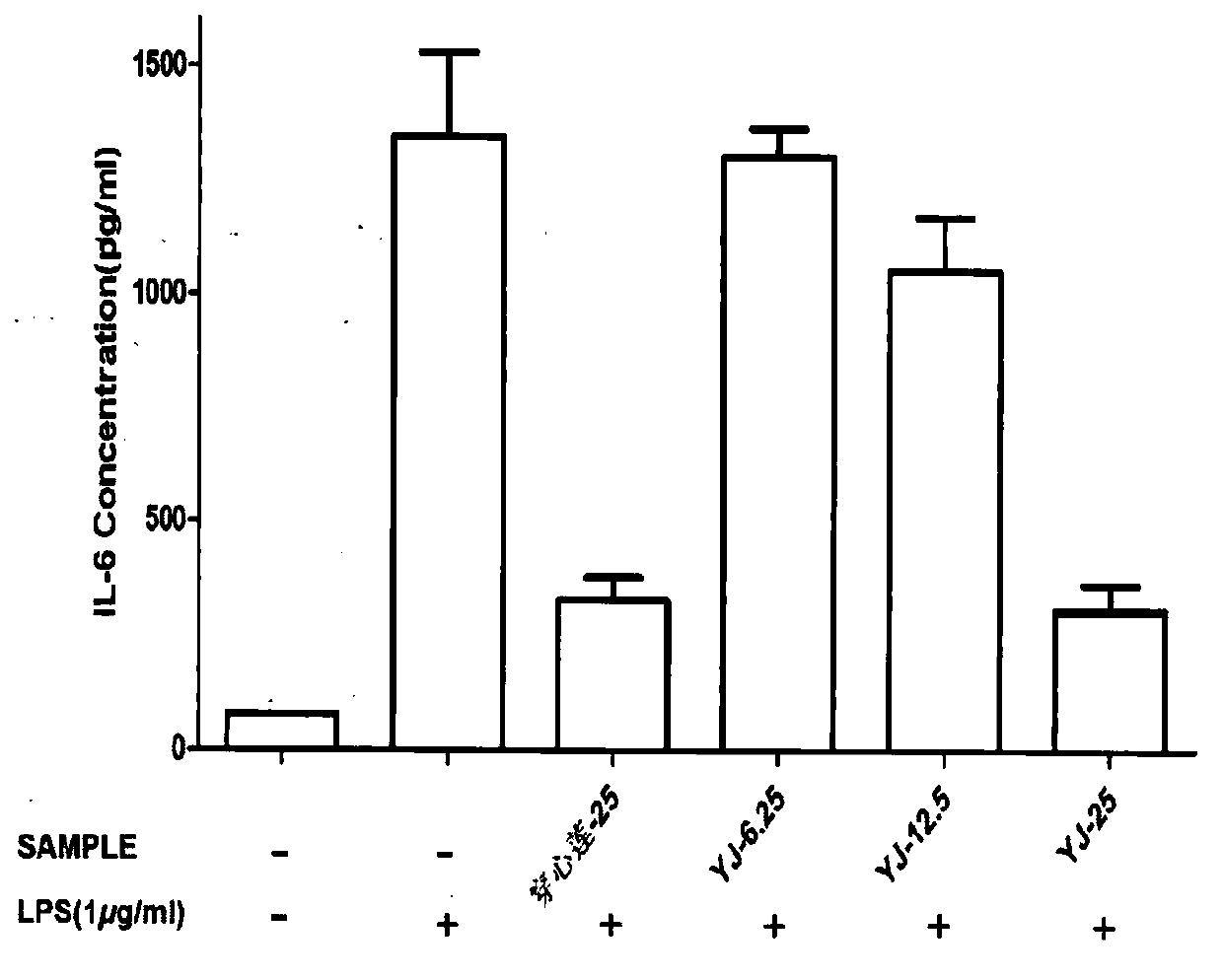
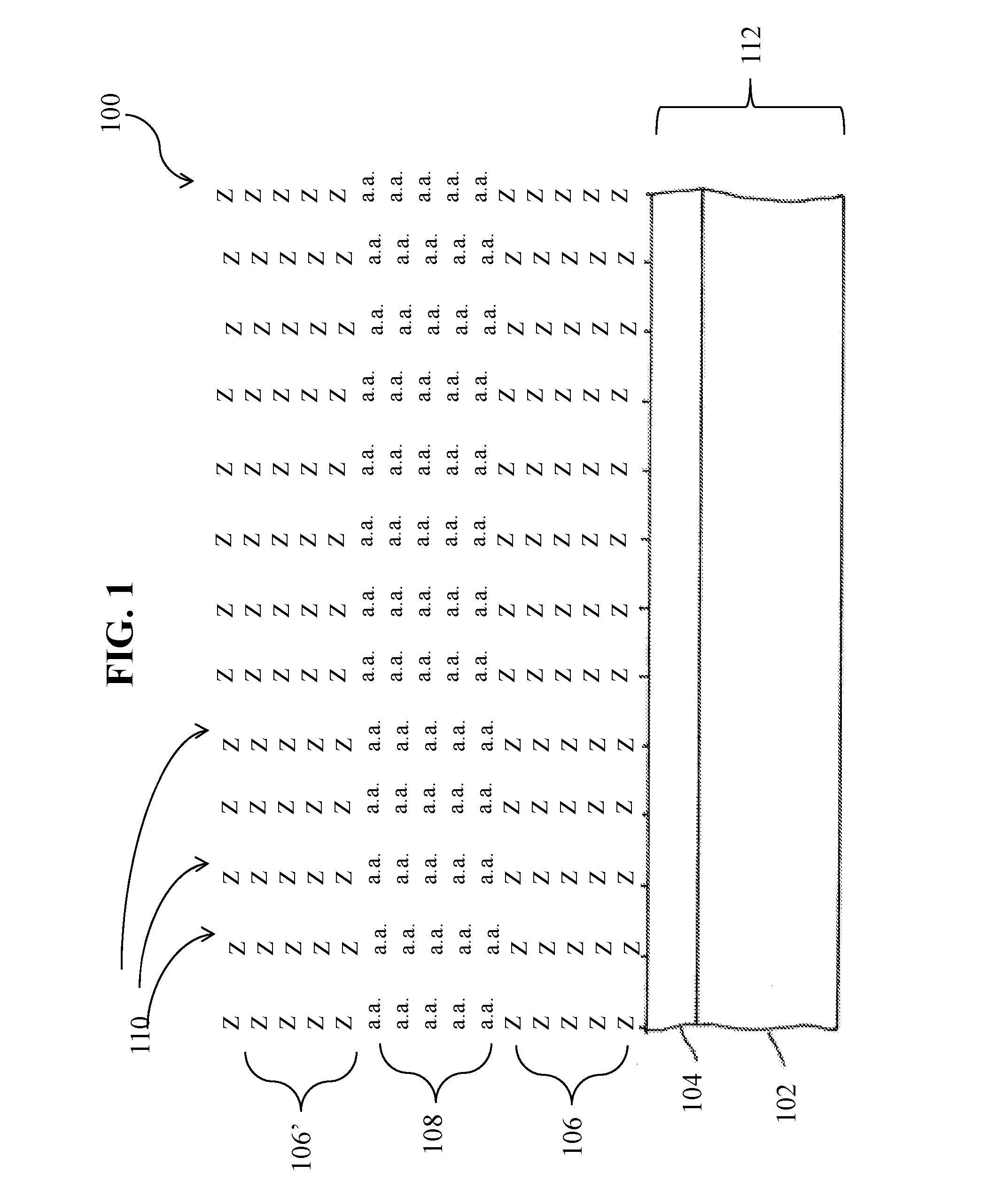
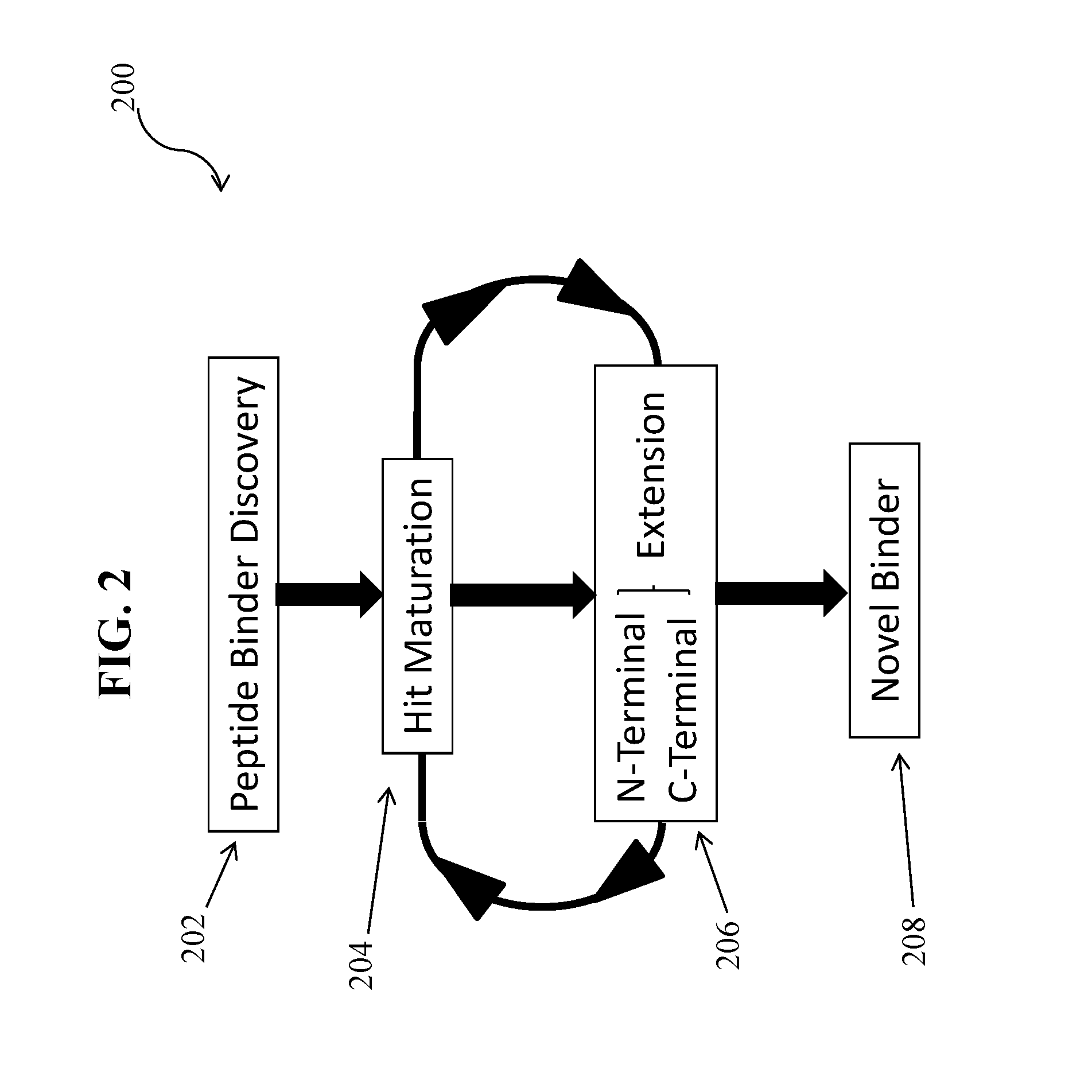
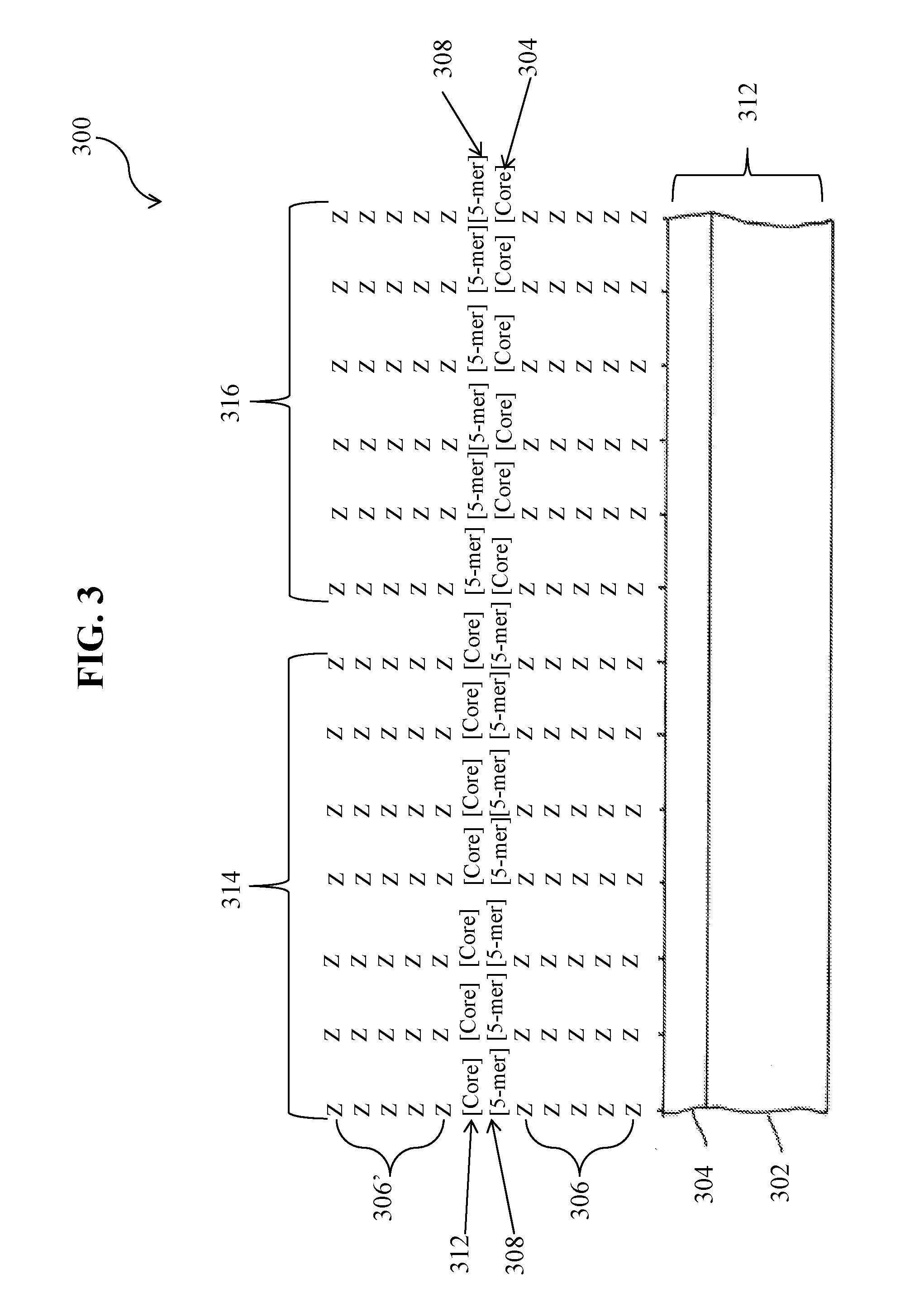
Specific peptide binders to proteins identified via systemic discovery, maturation and extension process
ActiveUS20160305952A1Biological material analysisPeptidesBiotin-streptavidin complexThrombin activity
The invention provides novel peptide binders for streptavidin (SA), Taq polymerase and several human proteins: Prostate Specific Antigen (PSA), thrombin, Tumor Necrosis Factor Alpha (TNFα), and Urokinase-type Plasminogen Activator (uPA).
Owner:ROCHE SEQUENCING SOLUTIONS INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com