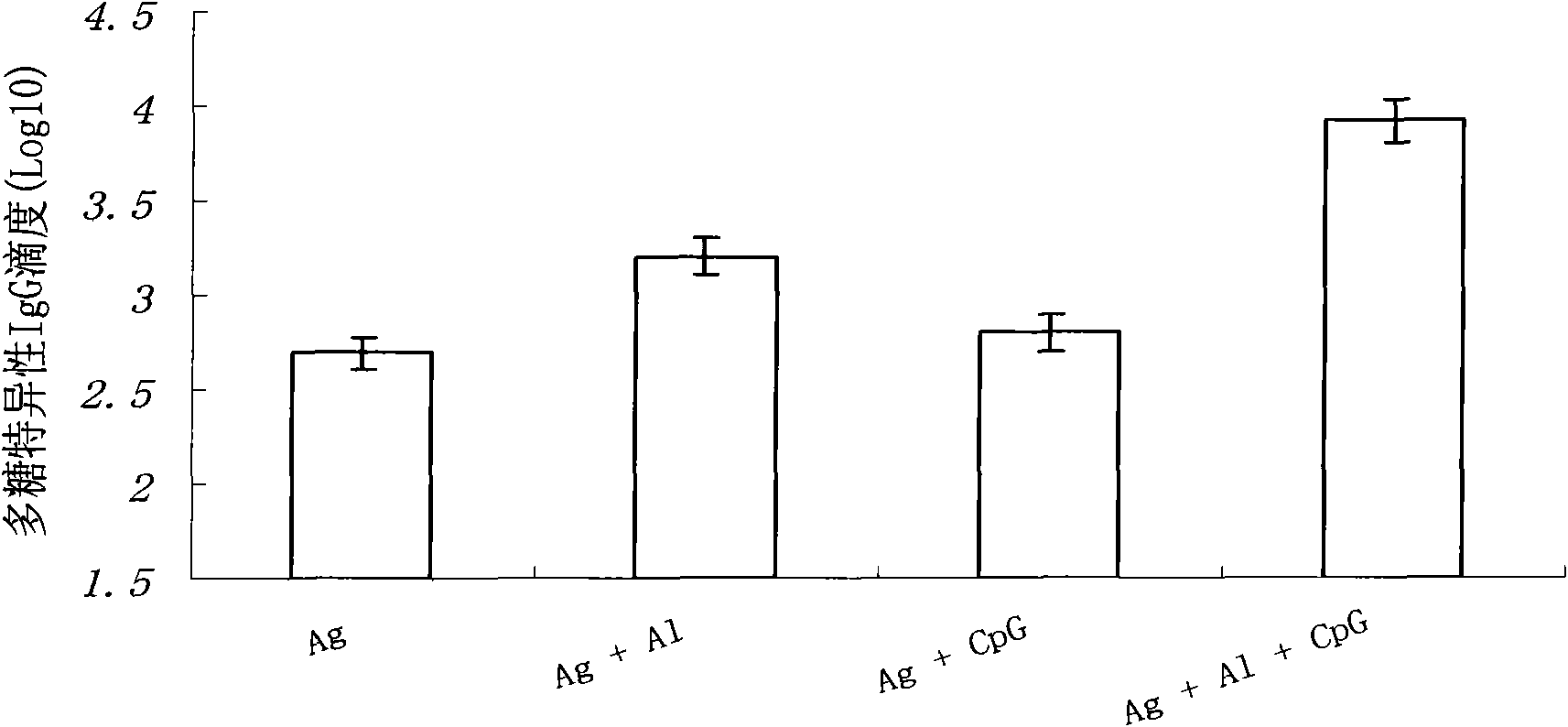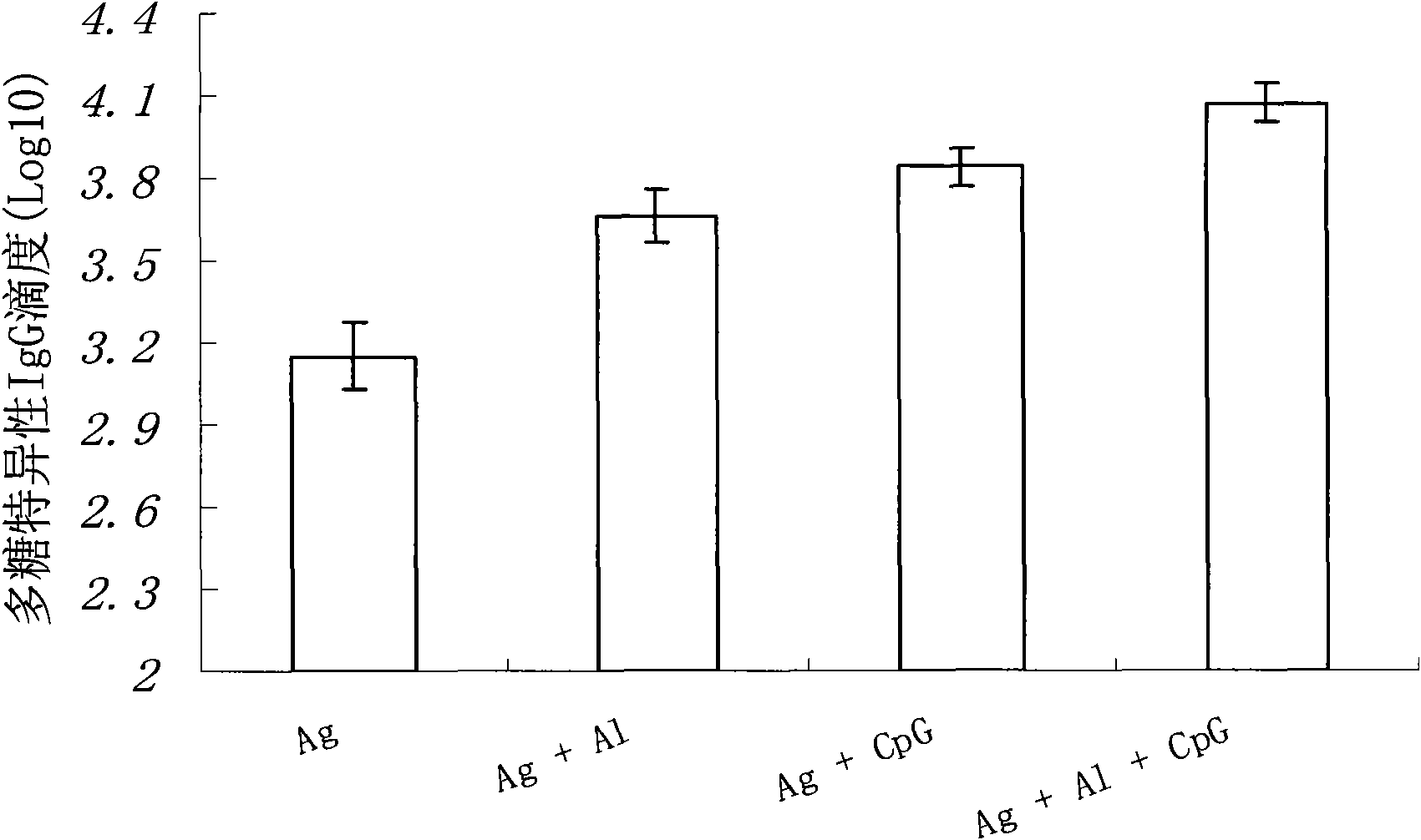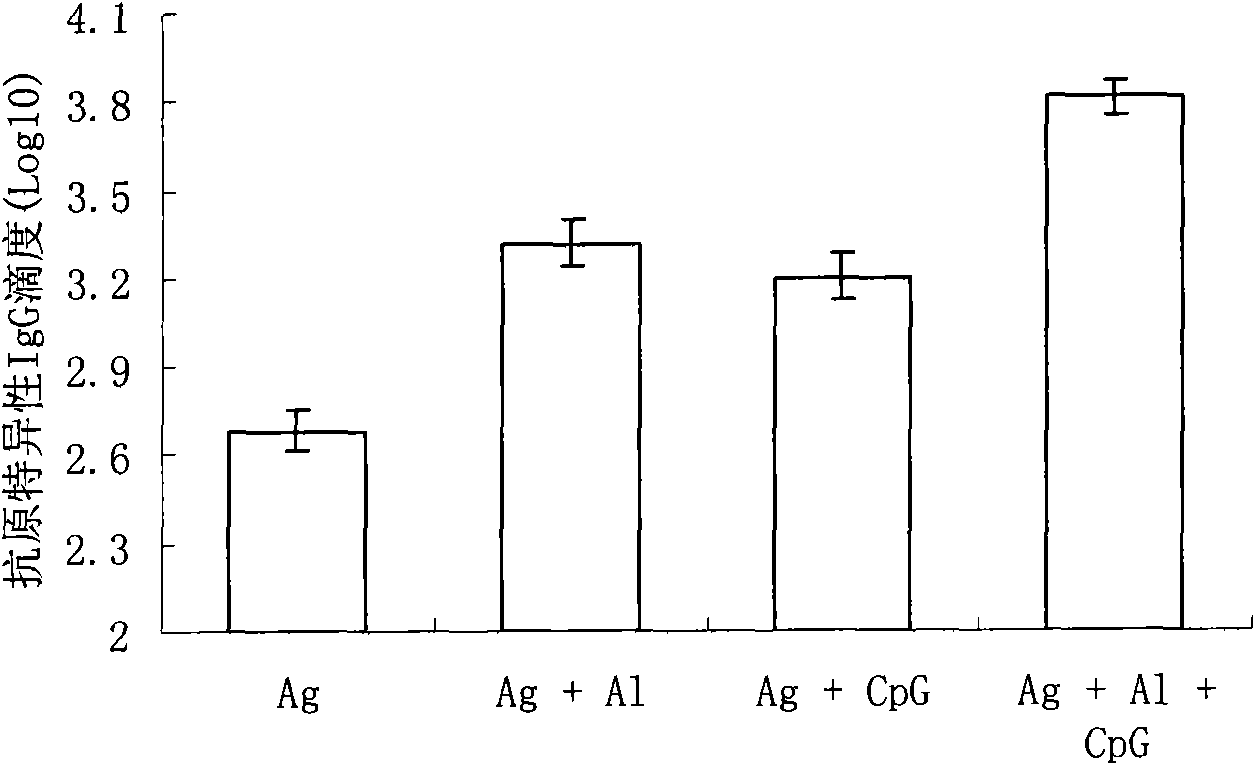Patents
Literature
106 results about "TLR9" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Toll-like receptor 9 is a protein that in humans is encoded by the TLR9 gene. TLR9 has also been designated as CD289 (cluster of differentiation 289). It is a member of the toll-like receptor (TLR) family. TLR9 is an important receptor expressed in immune system cells including dendritic cells, macrophages, natural killer cells, and other antigen presenting cells. TLR9 preferentially binds DNA present in bacteria and viruses, and triggers signaling cascades that lead to a pro-inflammatory cytokine response. Cancer, infection, and tissue damage can all modulate TLR9 expression and activation. TLR9 is also an important factor in autoimmune diseases, and there is active research into synthetic TLR9 agonists and antagonists that help regulate autoimmune inflammation.
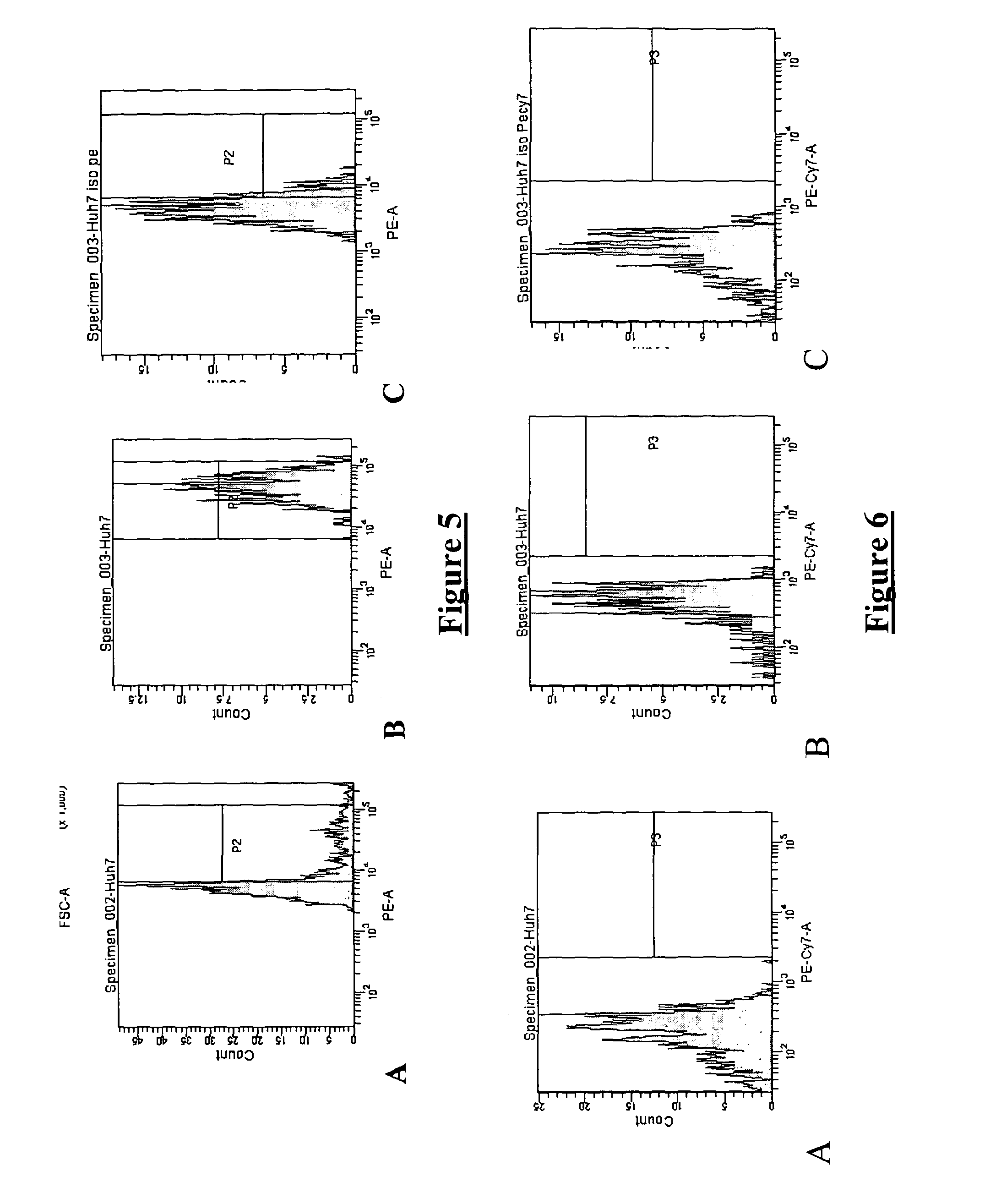
Treatment of cancer using tlr9 agonist with checkpoint inhibitors
InactiveUS20160101128A1Antitumor activityImprove permeabilityOrganic active ingredientsIn-vivo radioactive preparationsAgonistCancer research
Owner:IDERA PHARMA INC
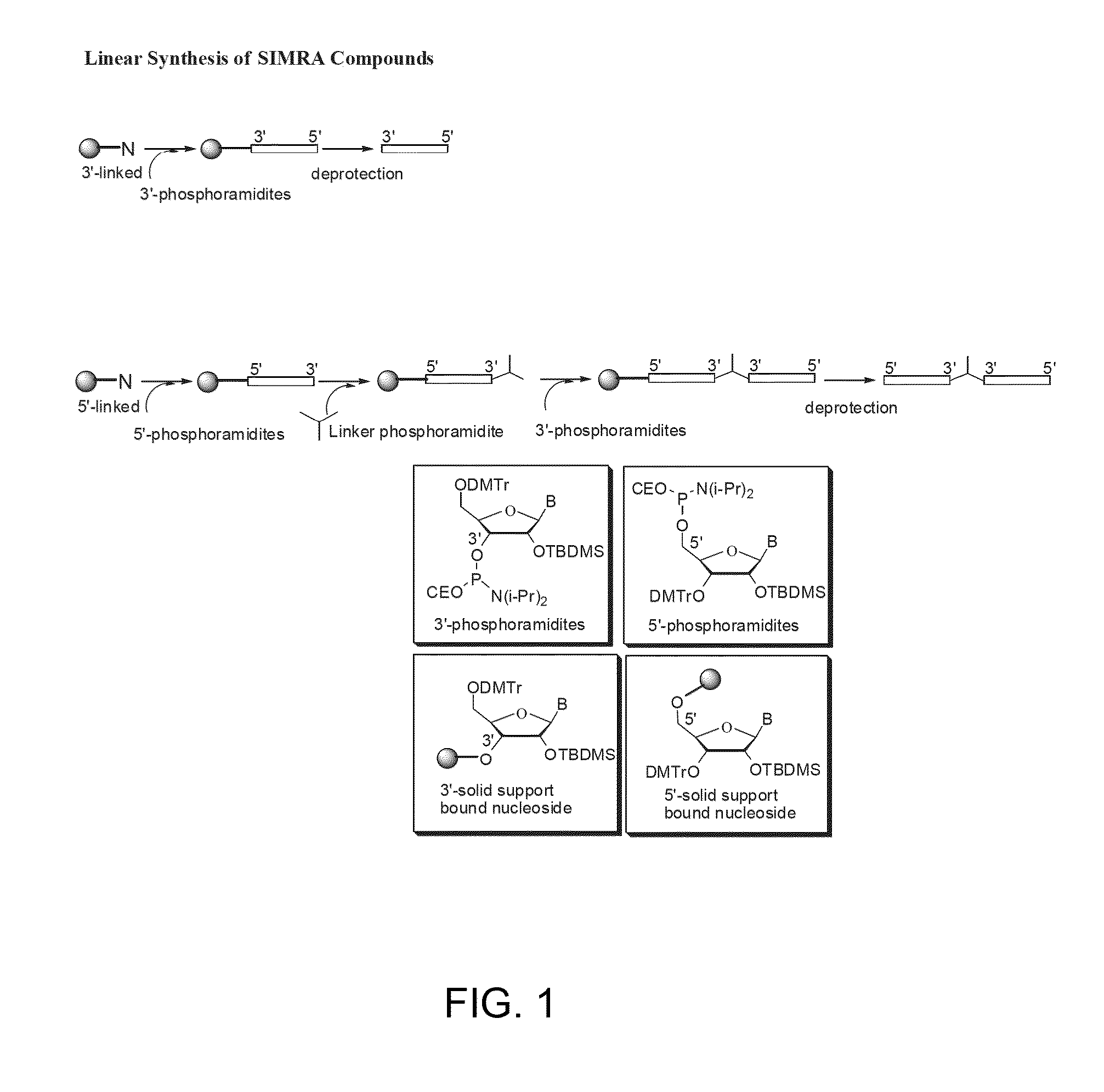
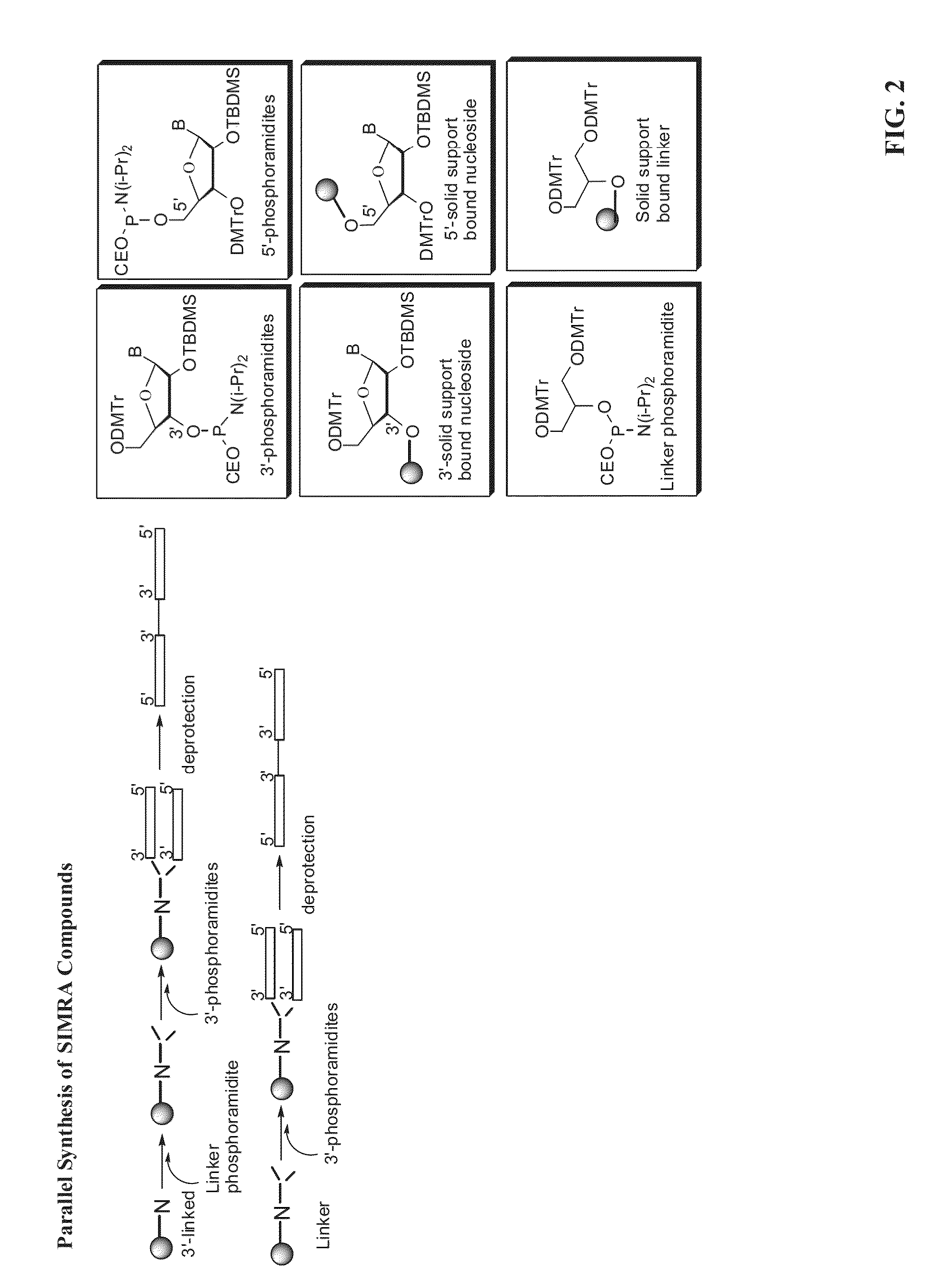
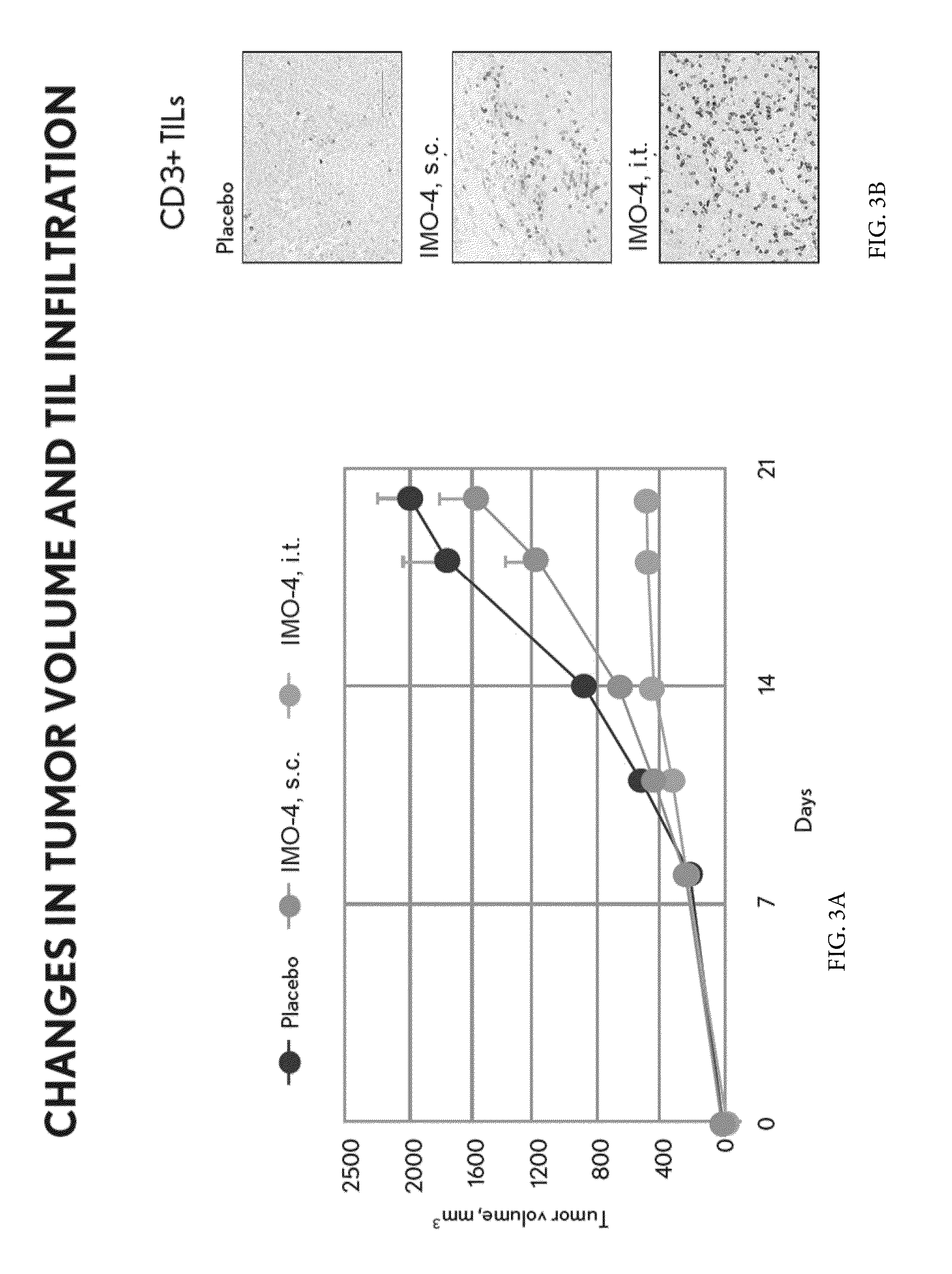
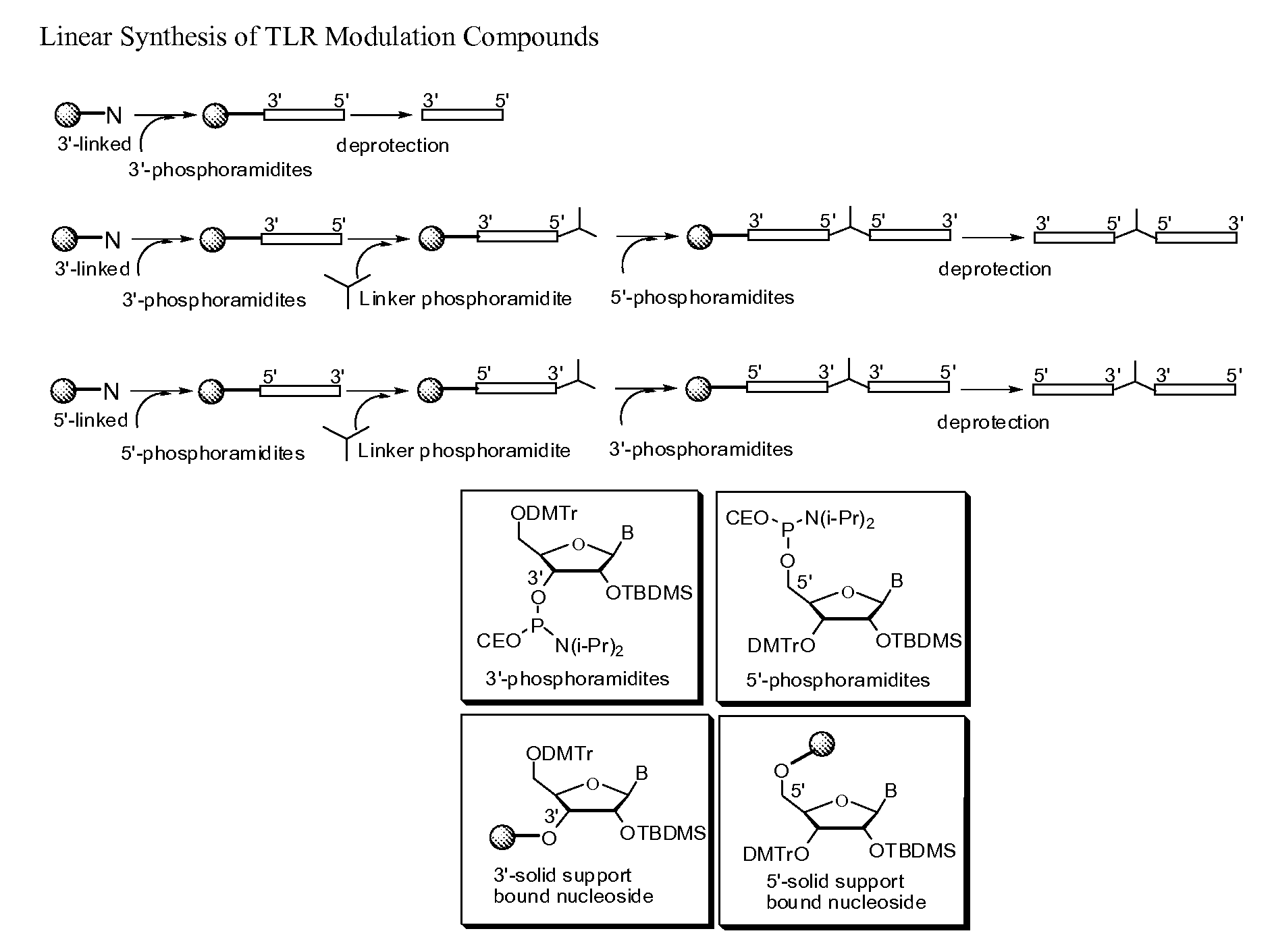
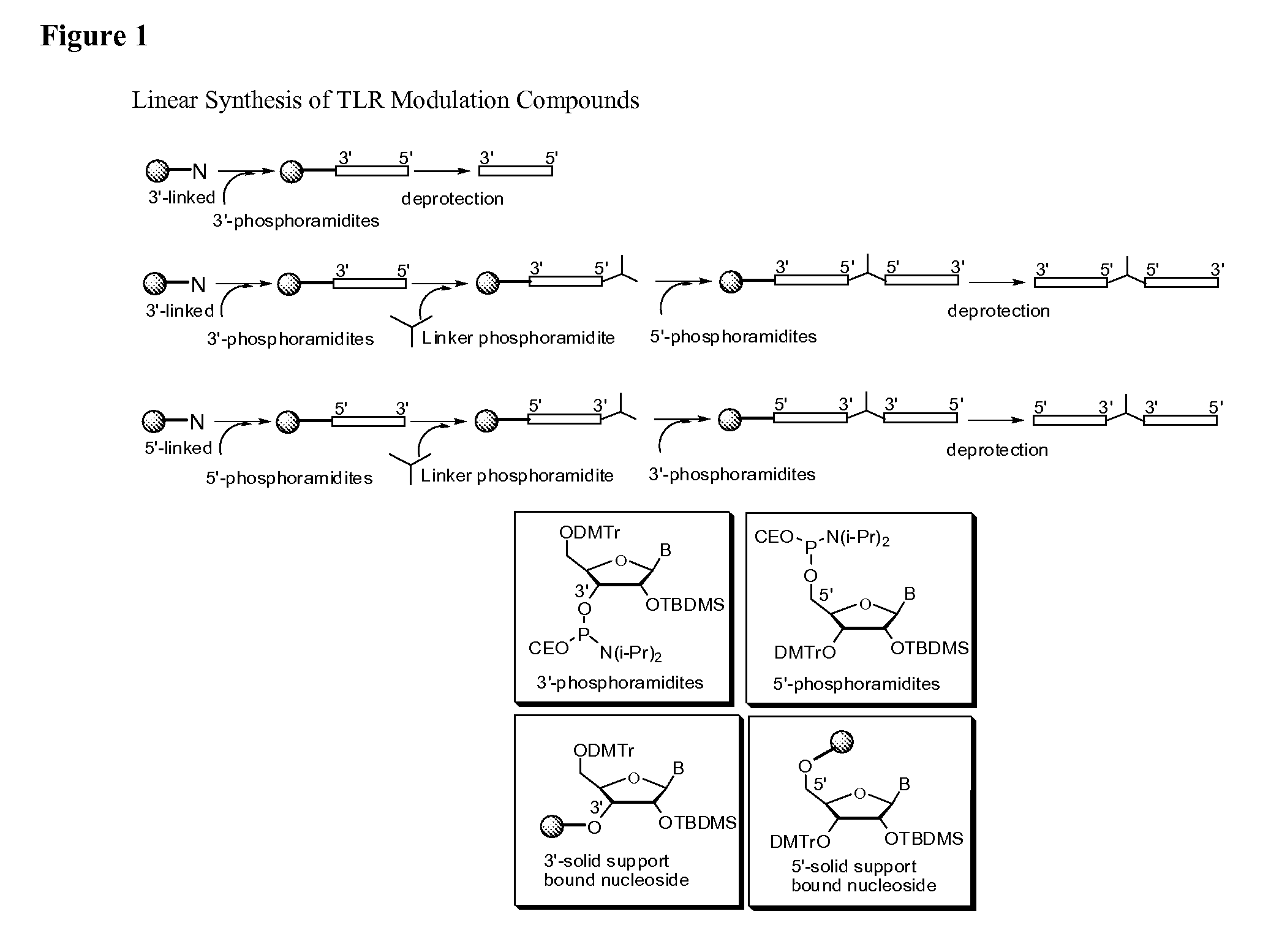
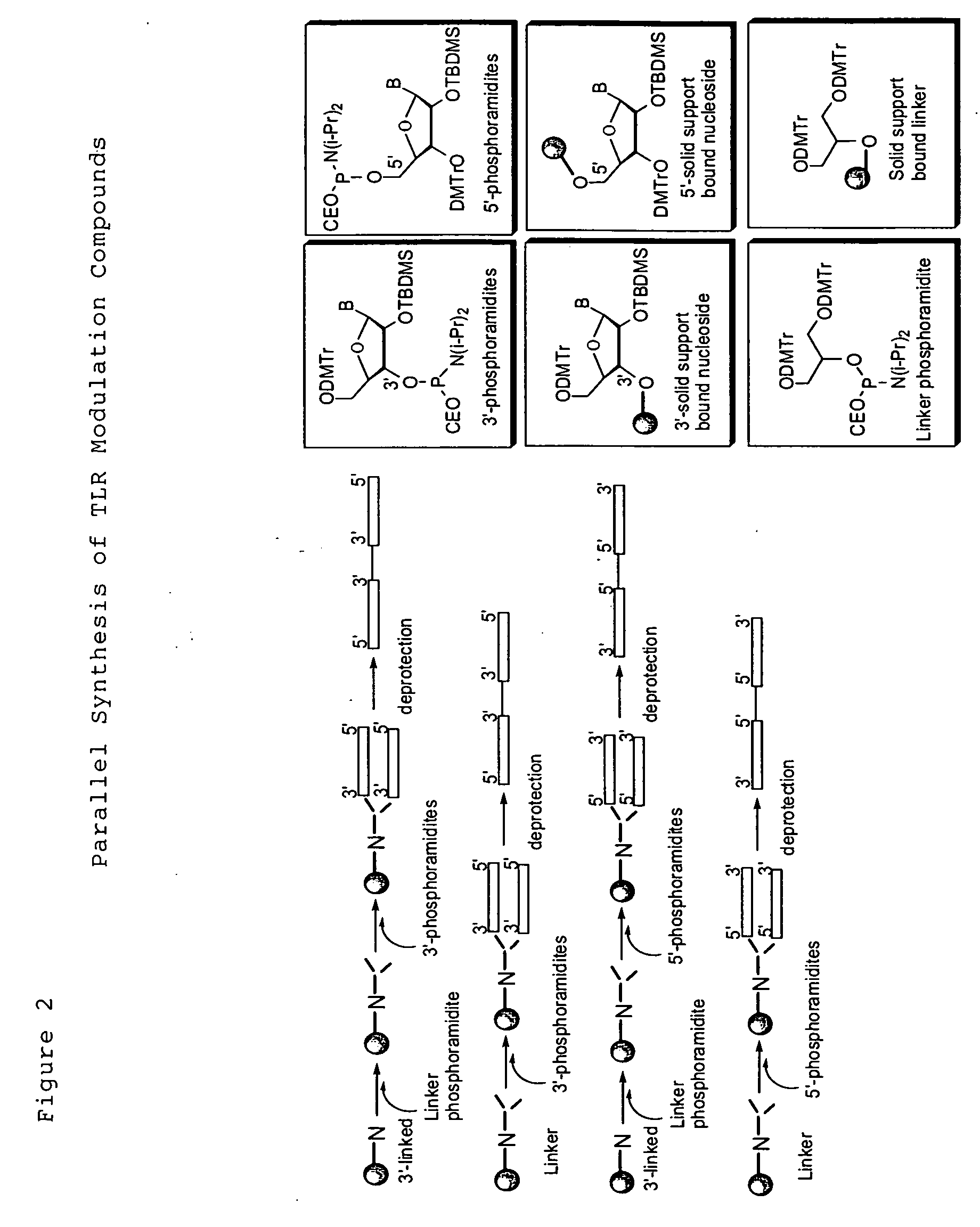
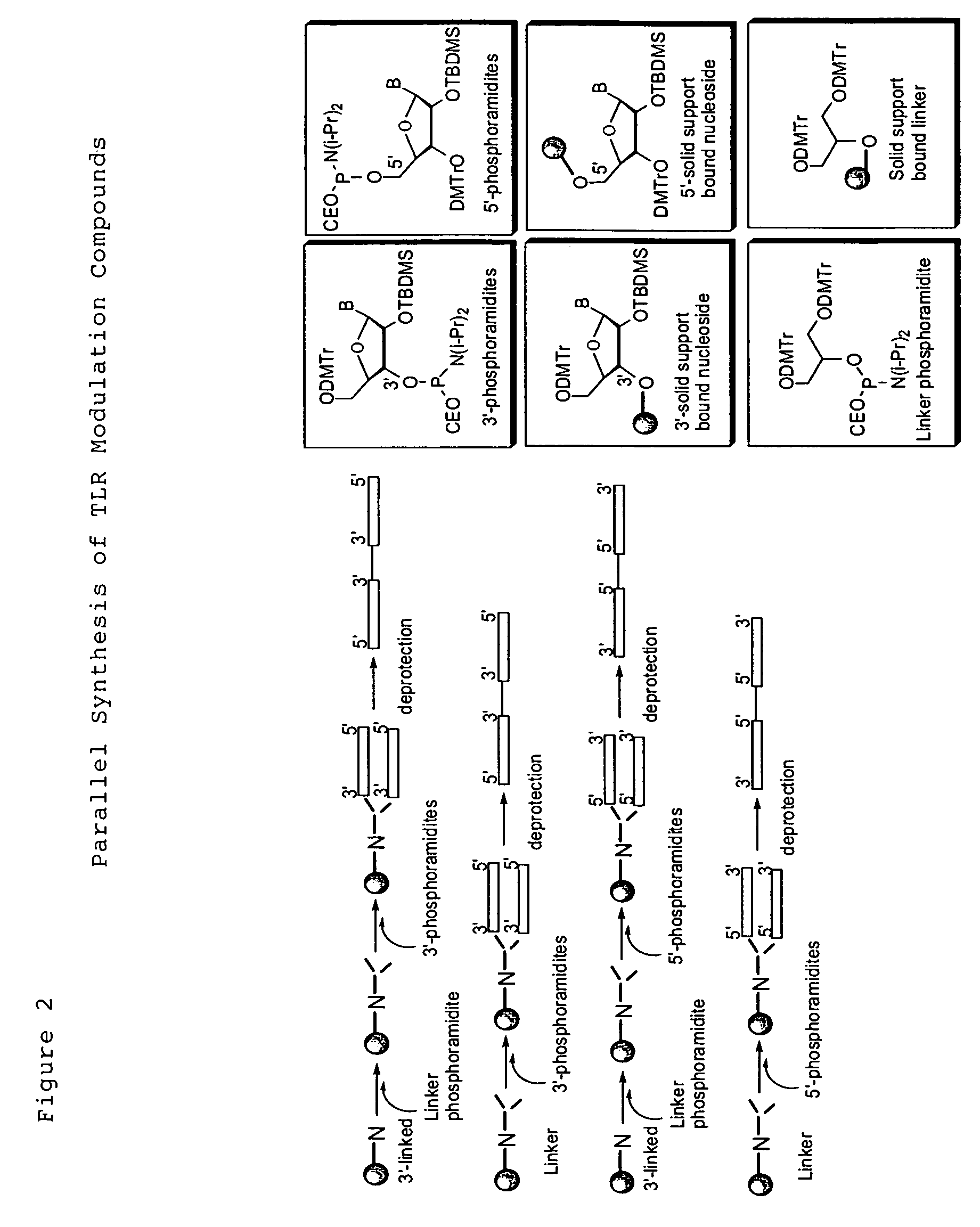
Toll like receptor modulators
ActiveUS20090053148A1Prevent diseaseLower immune responseSenses disorderNervous disorderInflammatory Bowel DiseasesAutoimmune responses
The invention relates to TLR9 antagonist compounds and their therapeutic or prophylactic use. The invention provides novel immune regulatory oligonucleotides and immunomers as antagonist of TLRs and methods of use thereof. These immune regulatory oligonucleotides have unique sequences that suppress, without completely ablating, TLR-mediated signaling in response to a TLR ligand or TLR signaling agonist. The methods may have use in the prevention and treatment of autoimmunity, inflammation, inflammatory bowel disease, lupus, allergy, asthma, infection, sepsis, cancer and immunodeficiency.
Owner:IDERA PHARMA INC
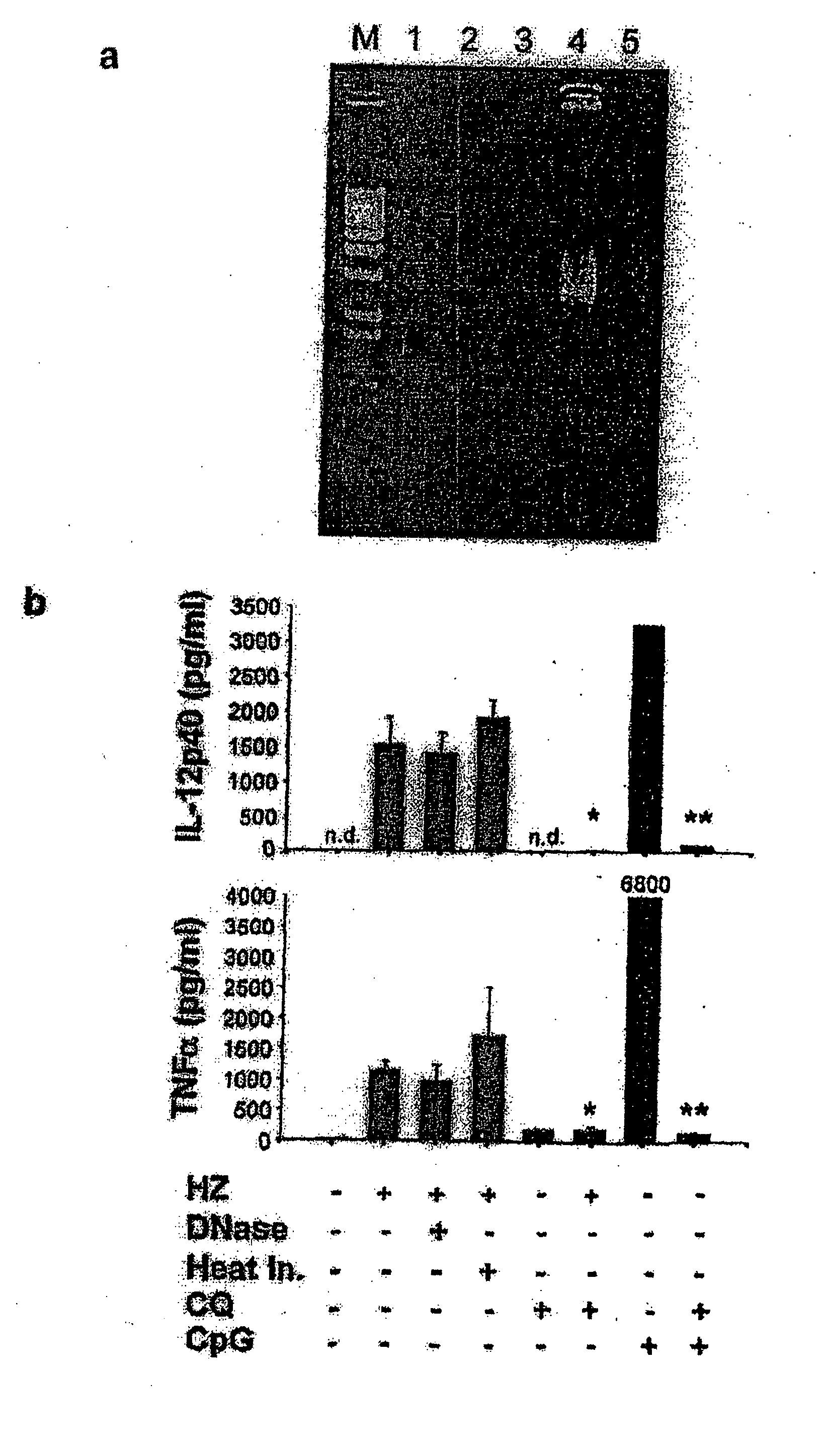
Detection/Measurement Of Malaria Infection Disease Utilizing Natural Immunity By Hemozoin Induction, Screening Of Preventative Or Therapeutic Medicine For Malaria Infection Disease, And Regulation Of Natural Immunity Induction
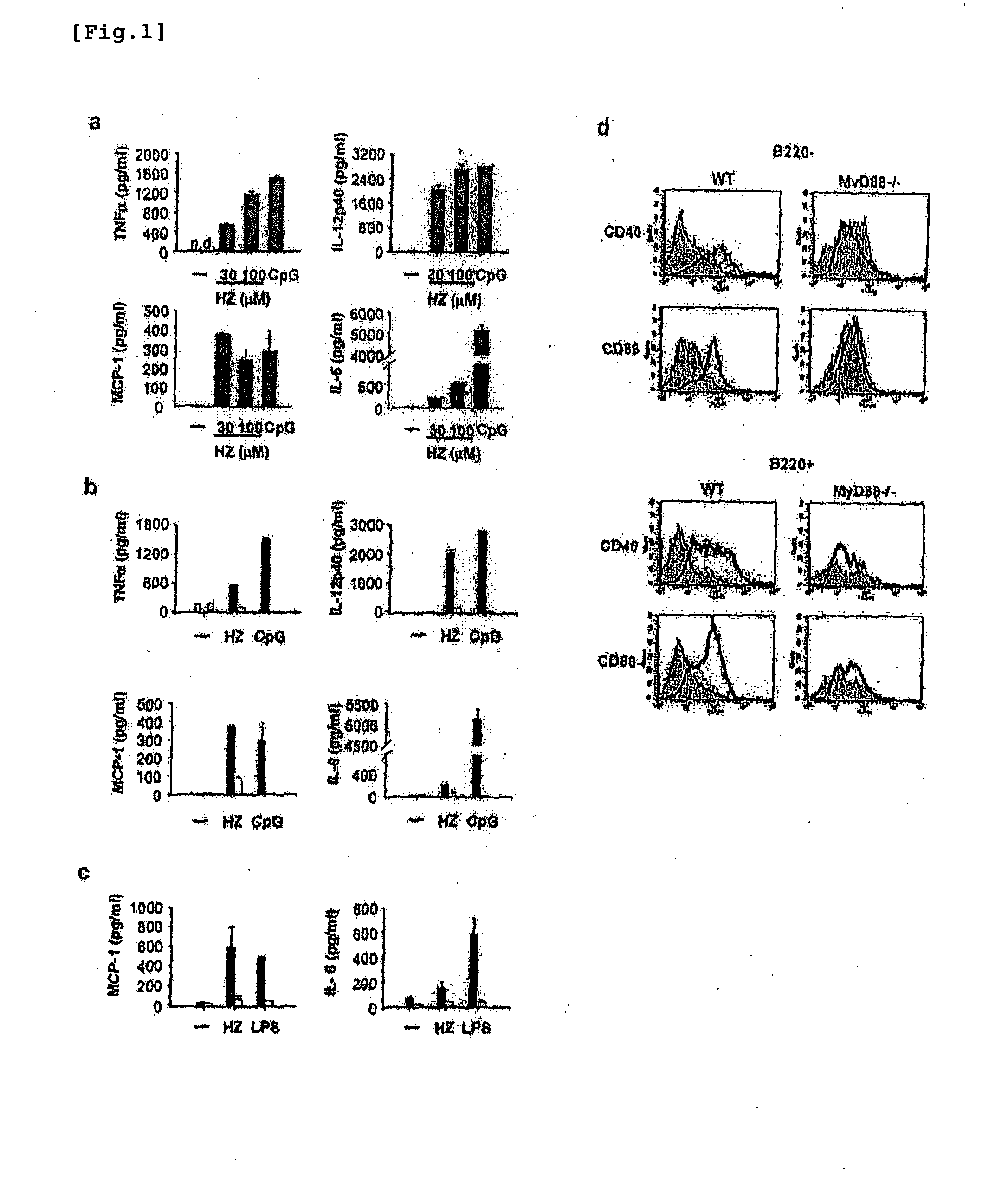
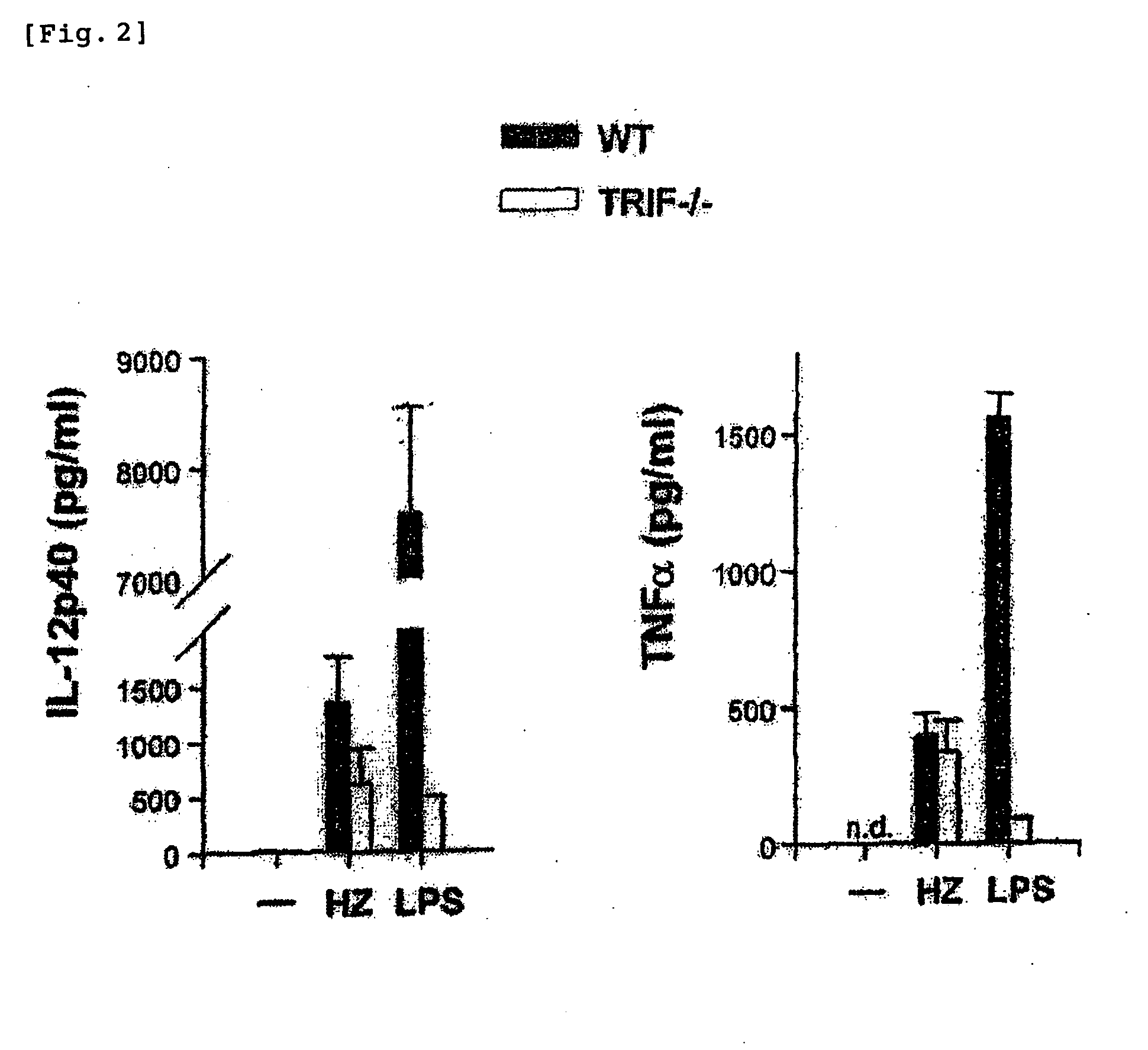
The instant invention is to provide a method for detecting and measuring malaria infection utilizing the induction by hemozoin (HZ); a method for screening a vaccine for malaria infection and a preventative or therapeutic agent for malaria infection using the method for detecting and measuring; and a means for regulating the induction of innate immunity using the HZ, synthetic HZ, or derivatives thereof as an adjuvant or immunostimulant. Malaria infection is detected and measured of by detecting and measuring HZ-induced, TLR9-mediated, and MyD88-dependent innate immune activity. The detection and measurement of malaria infection can be used to diagnose malaria infection. The method for detecting and measuring is also used for screening a vaccine for malaria infection and a preventative or therapeutic agent for malaria infection. Further, HZ, synthetic HZ, or derivatives thereof are used as an adjuvant or immunostimulant to regulate HZ-induced innate immune induction.
Owner:OSAKA UNIV
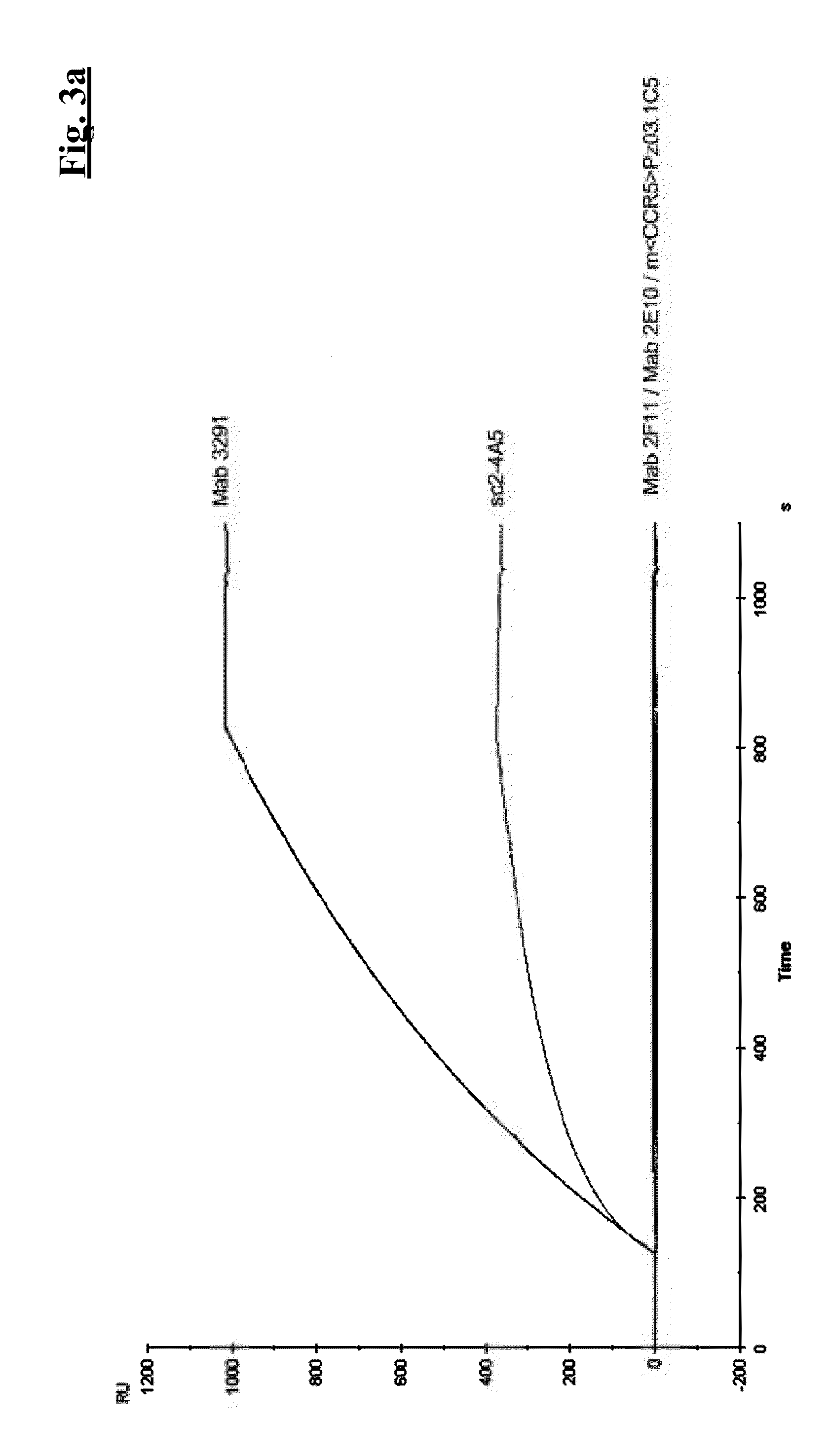
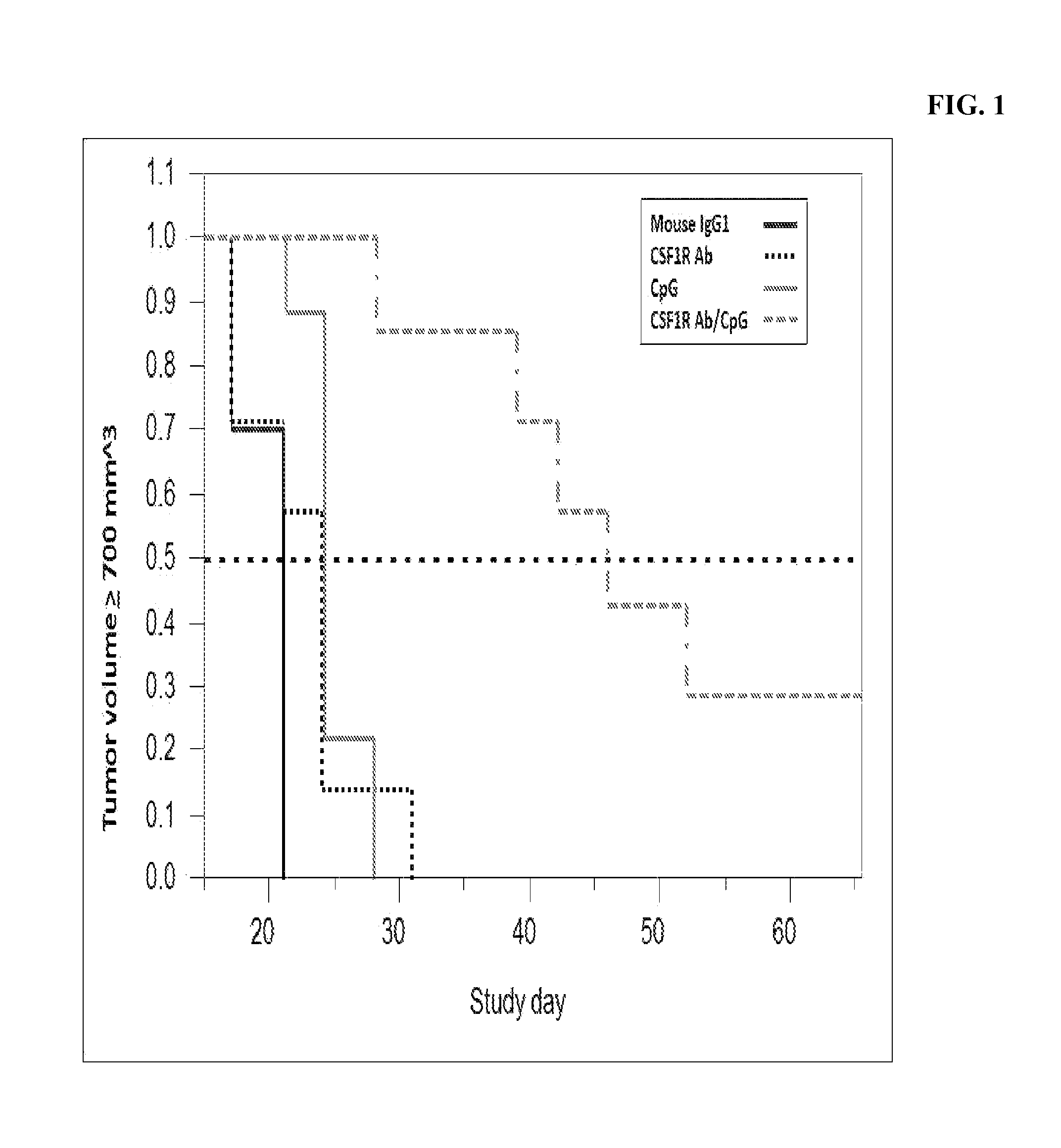
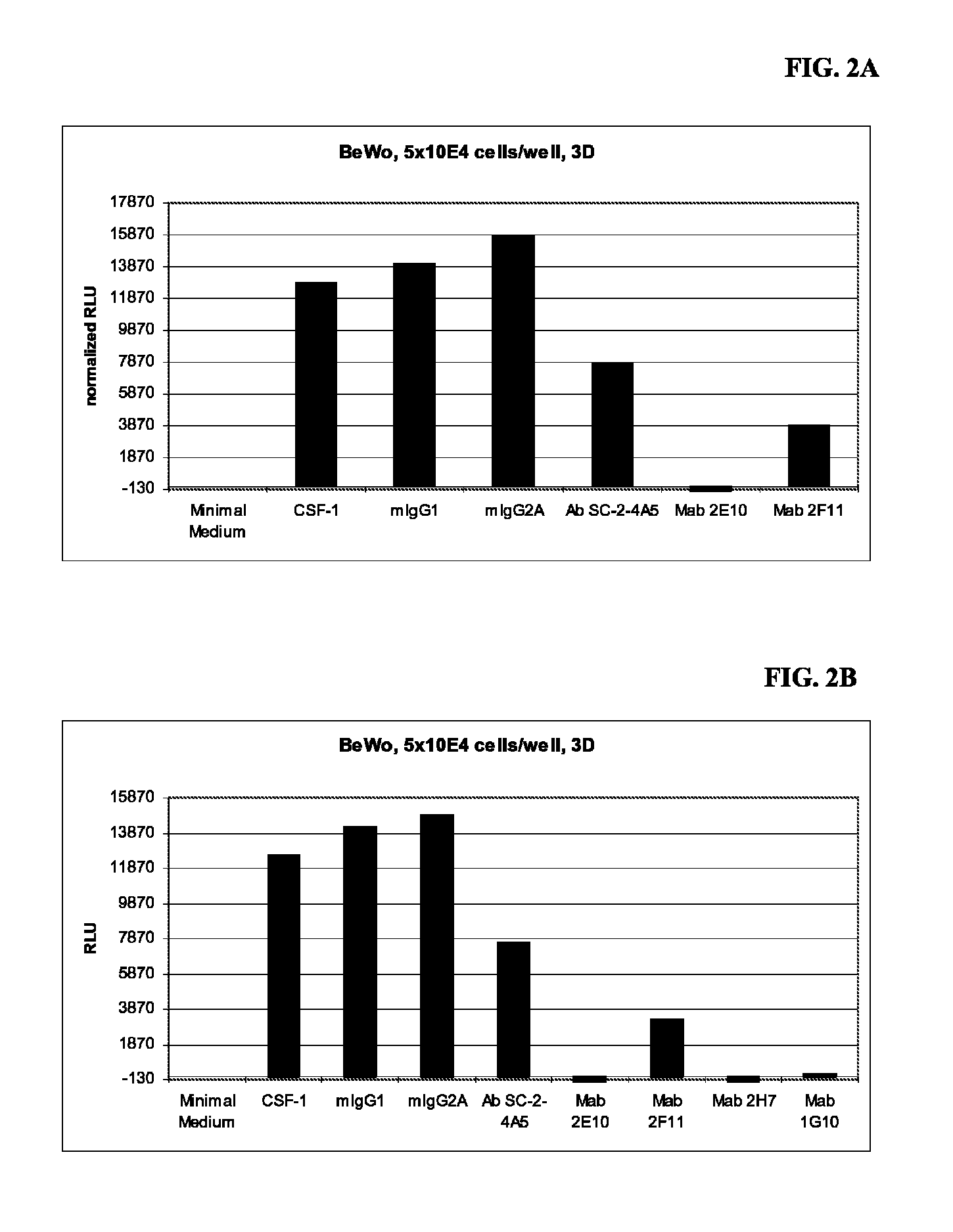
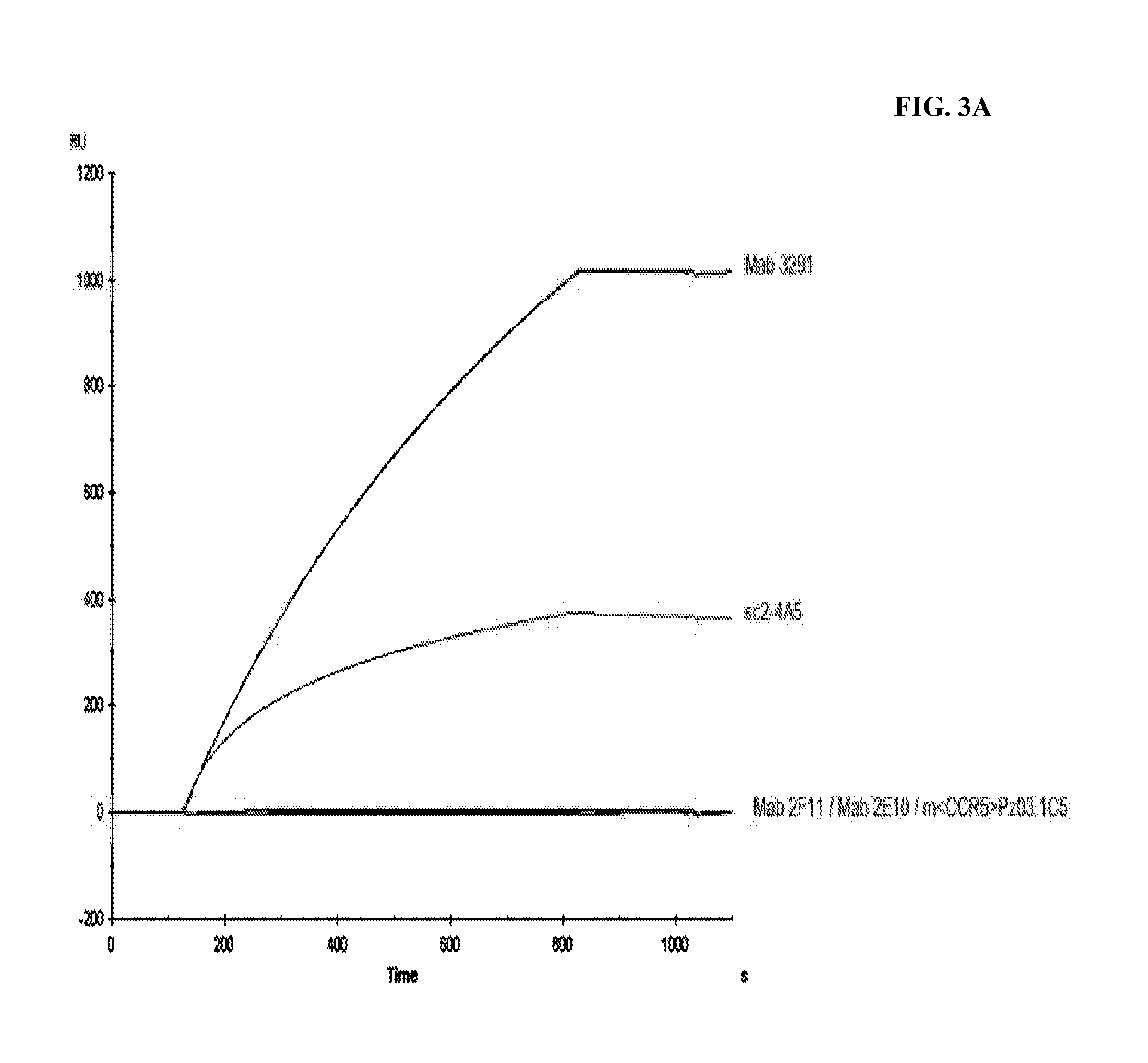
Combination therapy of antibodies against human csf-1r and tlr9 agonist
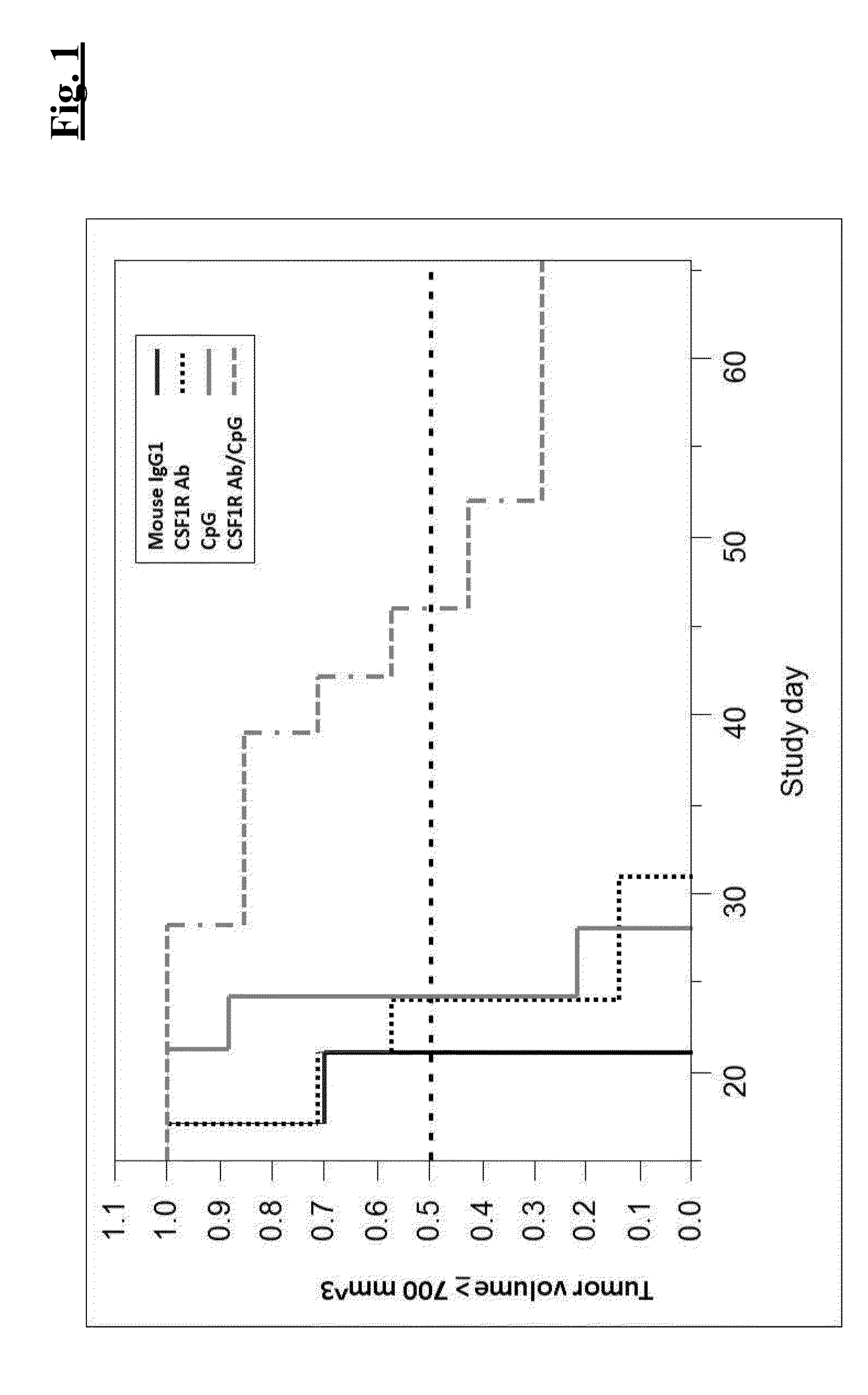
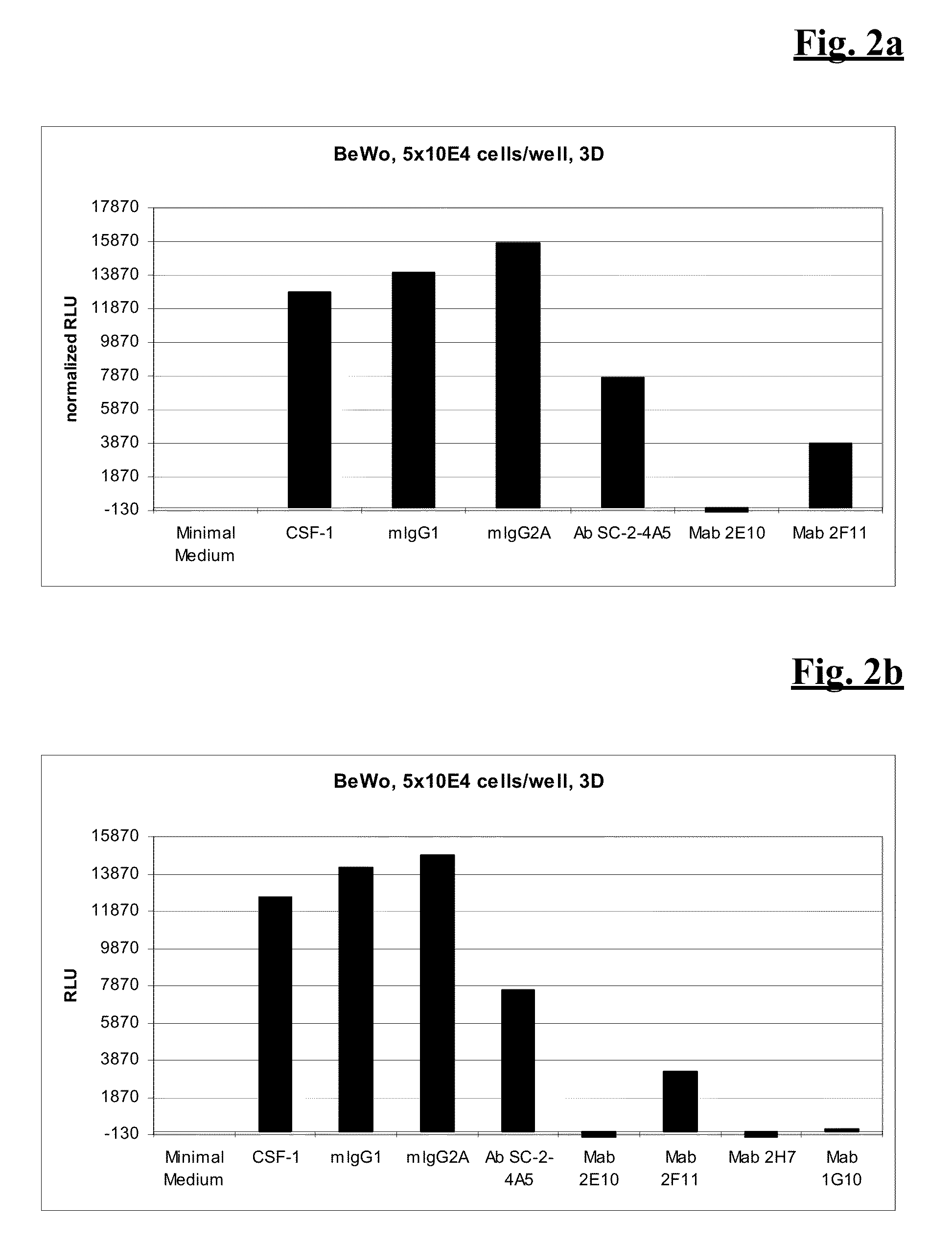
ActiveUS20140314771A1Effective activityUseful in treatment of cancerOrganic active ingredientsAntibody ingredientsAgonistCombination therapy
The present invention relates to the combination therapy of antibodies against human CSF-1R with a TLR9 agonist.
Owner:F HOFFMANN LA ROCHE & CO AG
Method of treating cancer by administering CSF-1R antibodies and a TLR9 agonist
ActiveUS9192667B2Effective activityUseful in treatment of cancerImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsAgonistCombination therapy
The present invention relates to the combination therapy of antibodies against human CSF-1R with a TLR9 agonist.
Owner:F HOFFMANN LA ROCHE & CO AG
Pyrrolo[3,2-c]pyridine derivatives as TLR inhibitors
InactiveUS9643967B2Useful in treatmentOrganic active ingredientsOrganic chemistryInflammatory Bowel DiseasesAutoimmune disease
The present invention provides a heterocyclic compound having a TLR7, TLR9, TLR7 / 8, TLR7 / 9 or TLR7 / 8 / 9 inhibitory action, which is useful as an agent for the prophylaxis or treatment of autoimmune diseases, inflammatory diseases and the like, in particular, systemic lupus erythematosus, Sjogren's syndrome, rheumatoid arthritis, psoriasis, inflammatory bowel disease and the like. The present invention is a compound represented by the formula (1): wherein each symbol is as described in the specification, or a salt thereof.
Owner:TAKEDA PHARMA CO LTD
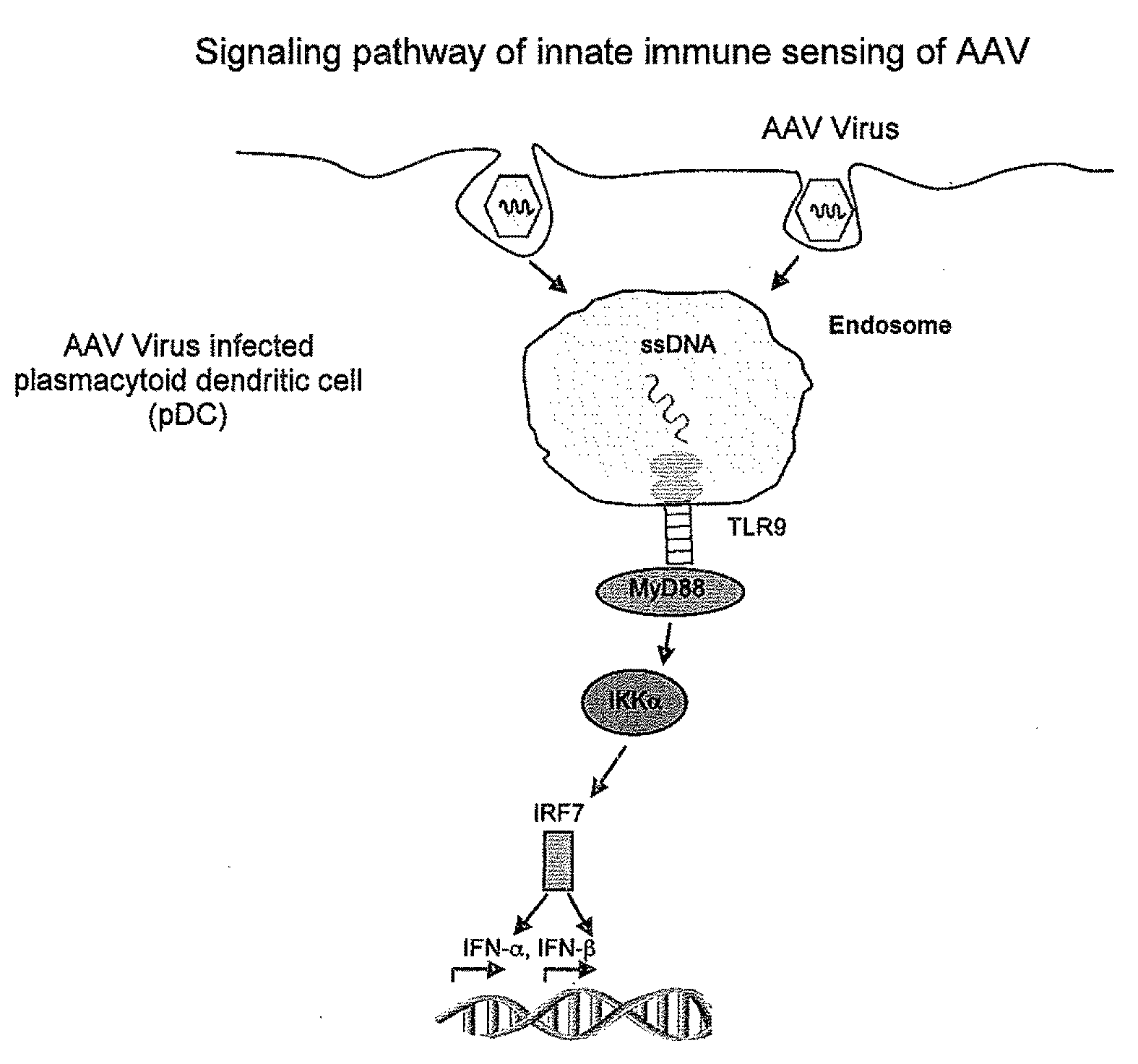
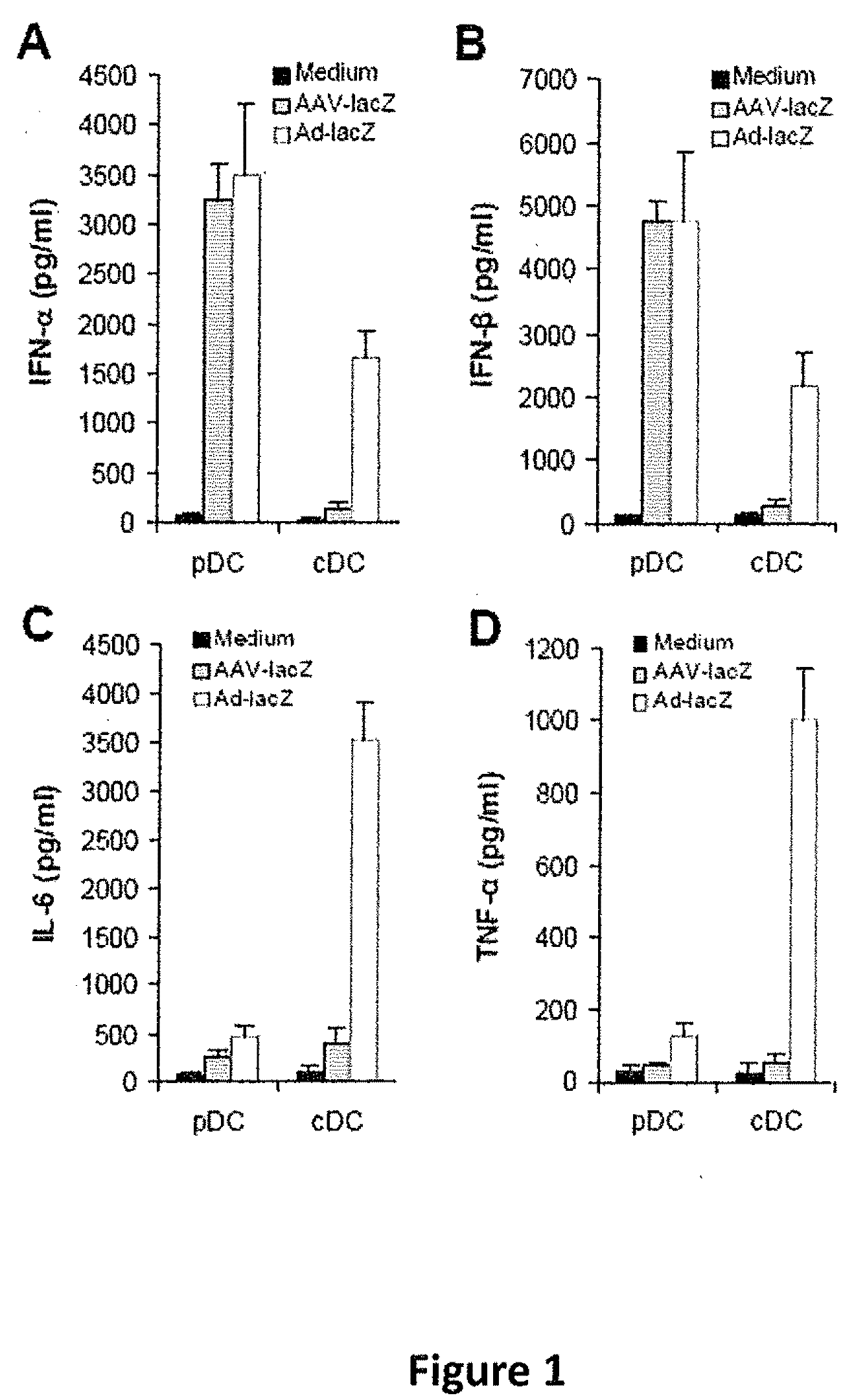
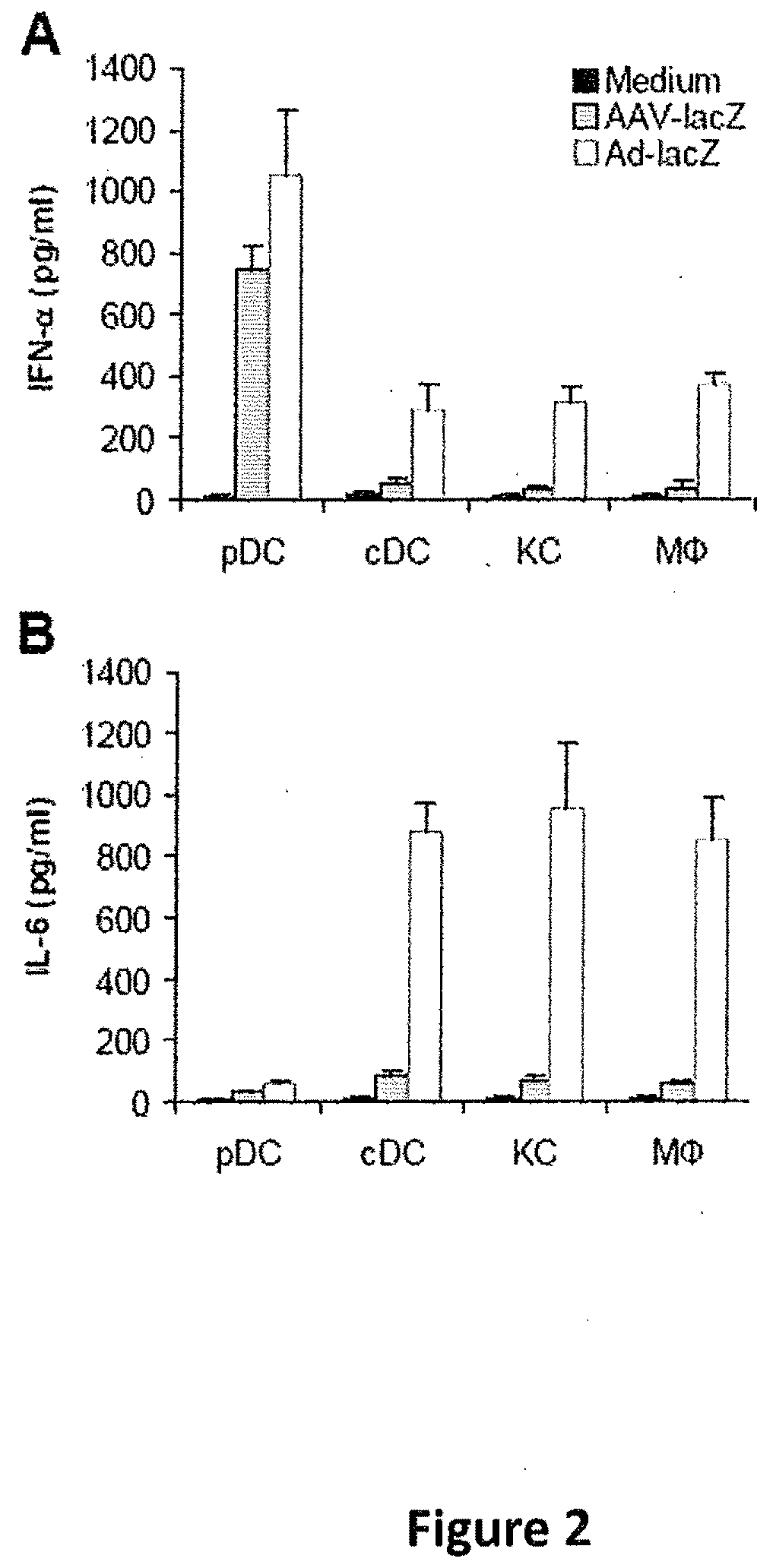
Methods for modulating immune responses to aav gene therapy vectors
InactiveUS20110070241A1Inhibits type I IFN productionDiminishes adaptive immune responseBiocideViral/bacteriophage medical ingredientsCo administrationViral vector
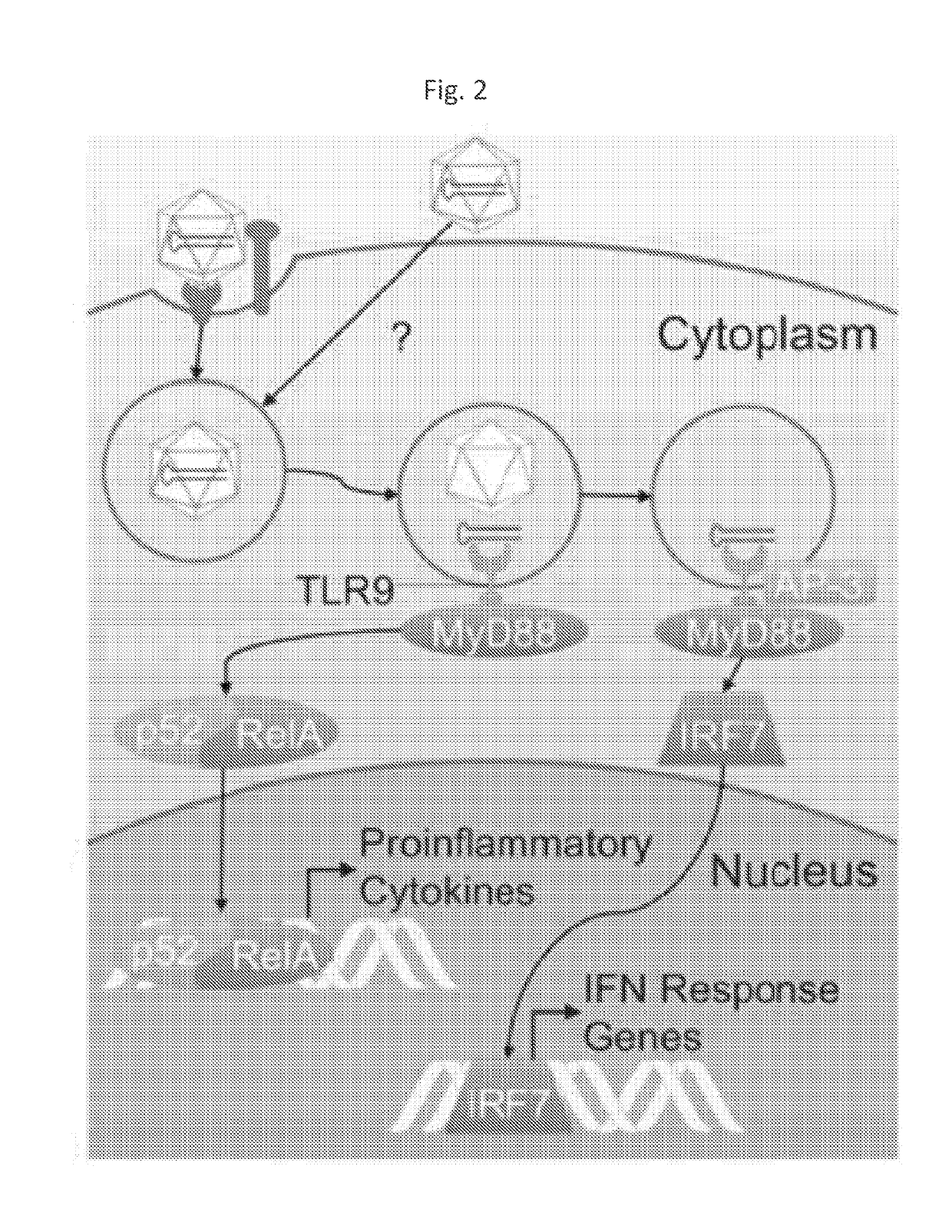
The present disclosure provides methods of inhibiting an immune response to a viral vector used in gene therapy, such as adeno-associated virus (AAV), which involves co-administration of viral vector and an interfering molecule. The interfering molecule functions by either disrupting the TLR9-MyD88-type I IFN signaling pathway and / or neutralizing Type I IFNs, thereby inhibiting the immune response directed against the viral vector. The methods additionally encompass the step of re-administering the viral vector.
Owner:DUKE UNIV
Toll like receptor modulators
ActiveUS8853375B2Prevent diseaseLower immune responseSenses disorderNervous disorderAutoimmune responsesAllergy
The invention relates to TLR9 antagonist compounds and their therapeutic or prophylactic use. The invention provides novel immune regulatory oligonucleotides and immunomers as antagonist of TLRs and methods of use thereof. These immune regulatory oligonucleotides have unique sequences that suppress, without completely ablating, TLR-mediated signaling in response to a TLR ligand or TLR signaling agonist. The methods may have use in the prevention and treatment of autoimmunity, inflammation, inflammatory bowel disease, lupus, allergy, asthma, infection, sepsis, cancer and immunodeficiency.
Owner:IDERA PHARMA INC
Regulation of toll-like receptors on stem cells
InactiveUS7592003B2Reduce expressionReduce functionBiocideMammal material medical ingredientsProgenitorHematopoietic cell
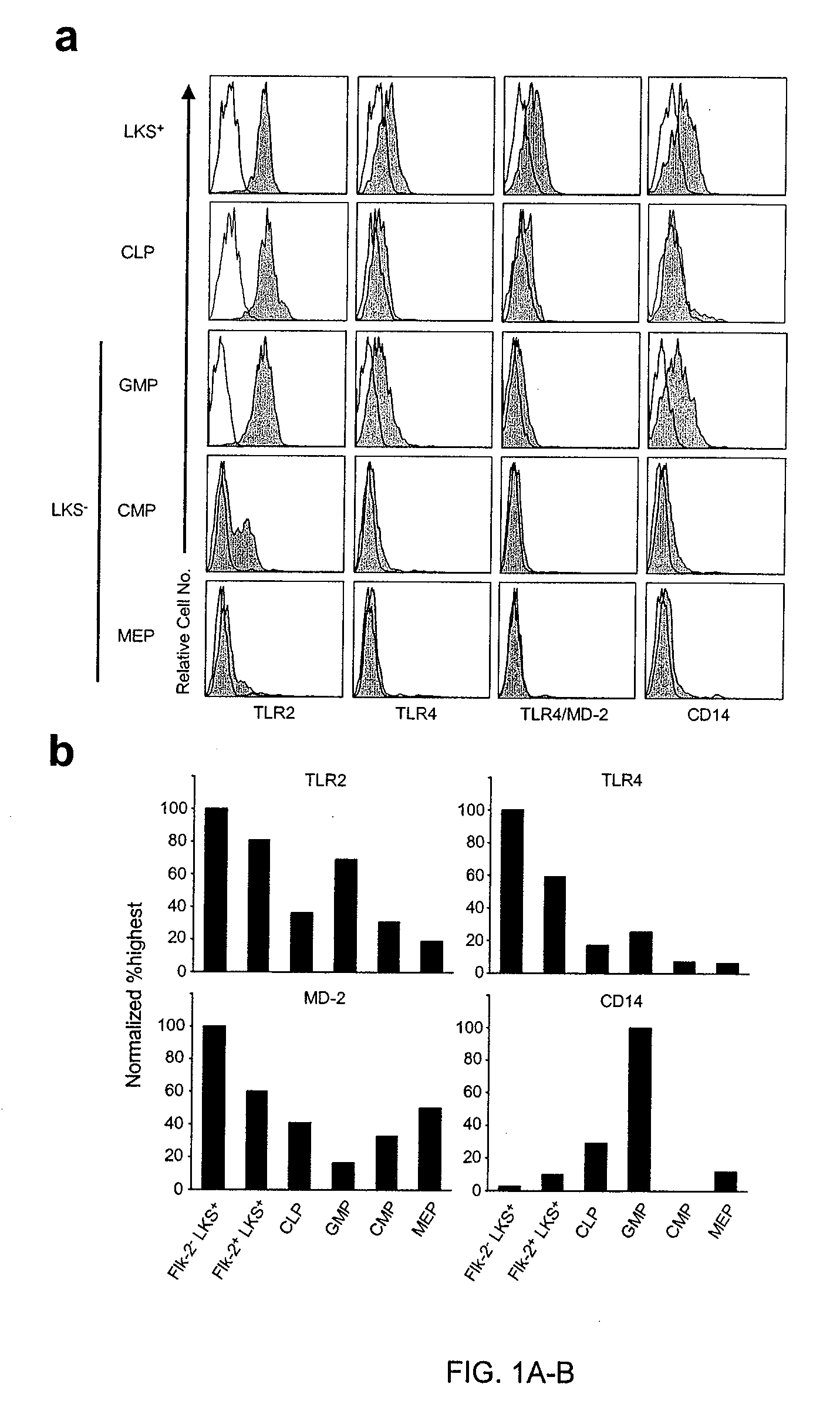
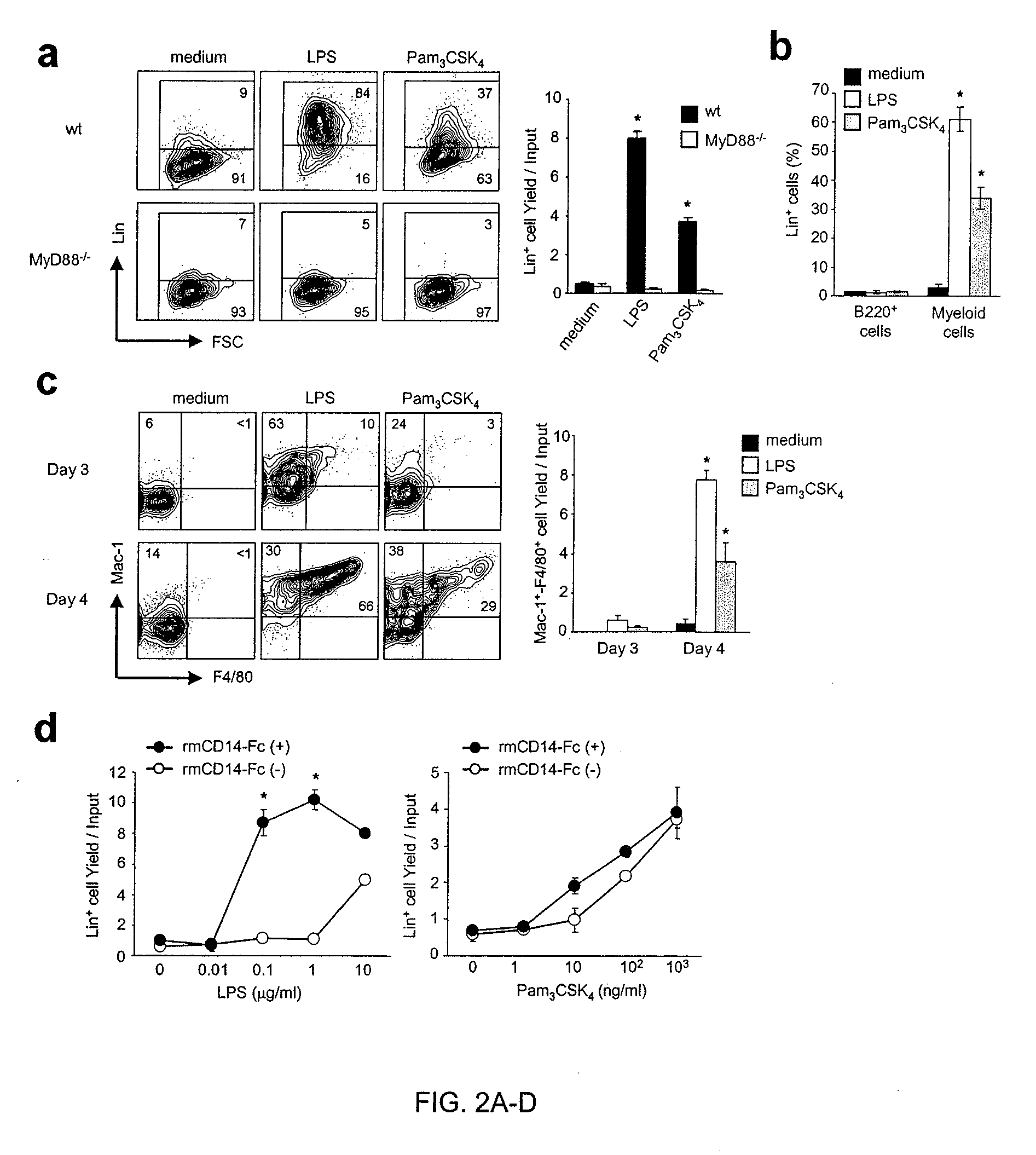
The discovery of Toll-like receptors (TLRs) on the surface of hematopoietic cells provides new methods for the stimulation and differentiation of various classes of progenitor cells. TLR2 and TLR4 agonists (natural ligands, mimetics, antibodies) are particularly useful in these methods. The cells can be isolated and used for various purpose including tissue regeneration and grafting. In contrast, antagonists of TLRs can be used to protect cells from various insults such as chemo- and radiotherapy, acute and chronic infection, and transplantation by inhibiting activation and differentiation. TLR2, TLR4 and TLR9 pathway antagonists (soluble TLR, mimetics, antibodies) are particularly useful in these methods. Cells can be isolated and used for various purposes including transplantation.
Owner:OKLAHOMA MEDICAL RES FOUND
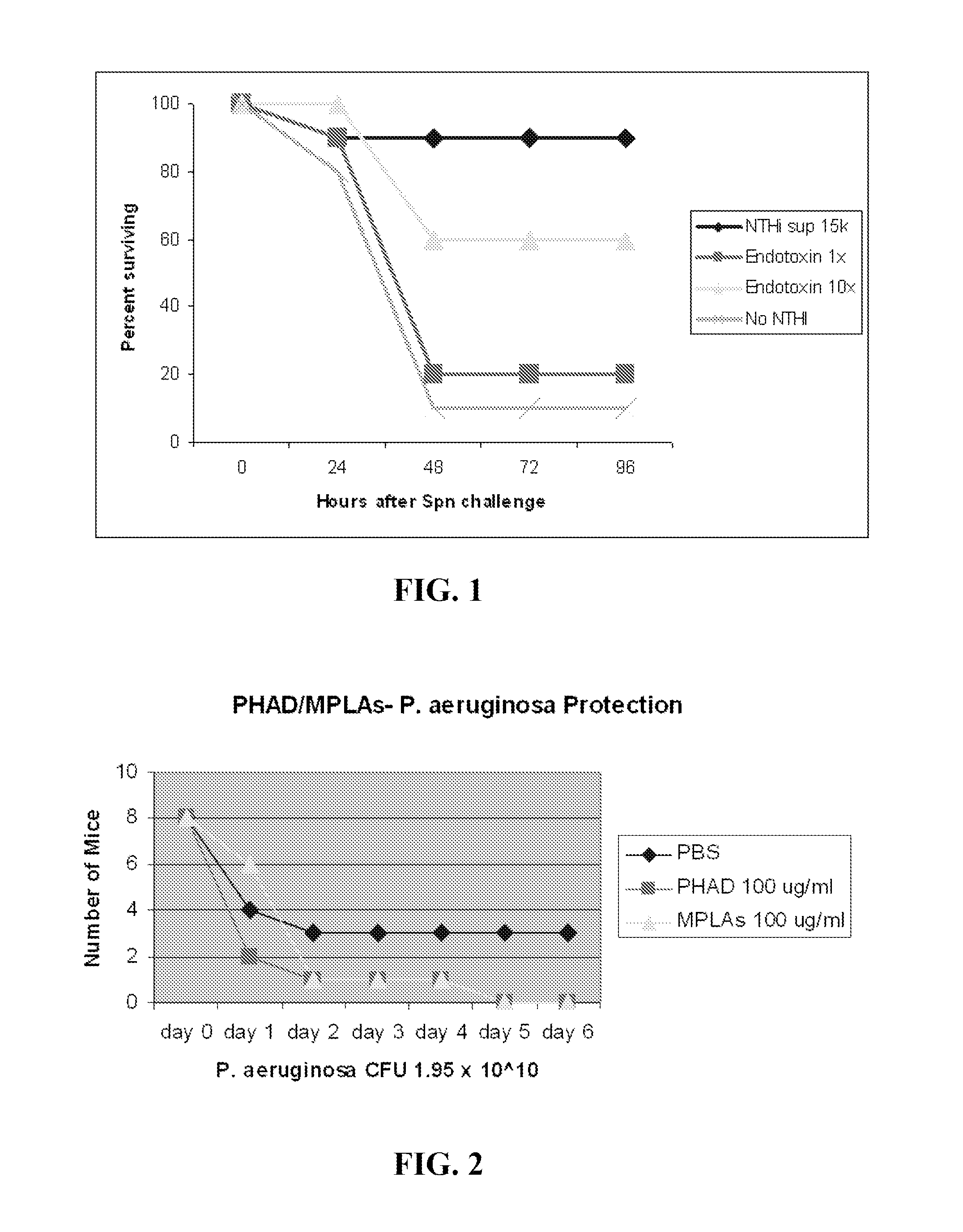
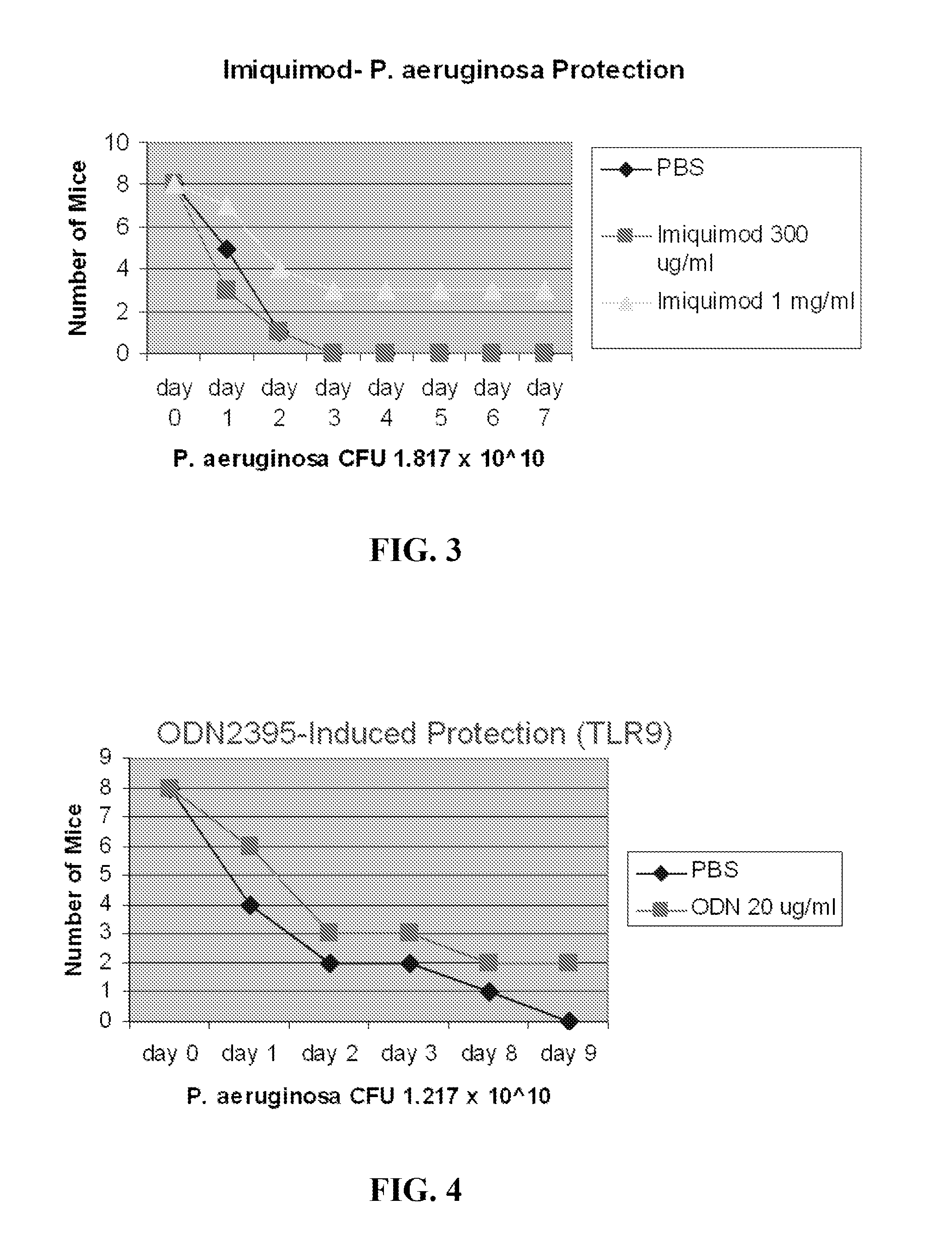
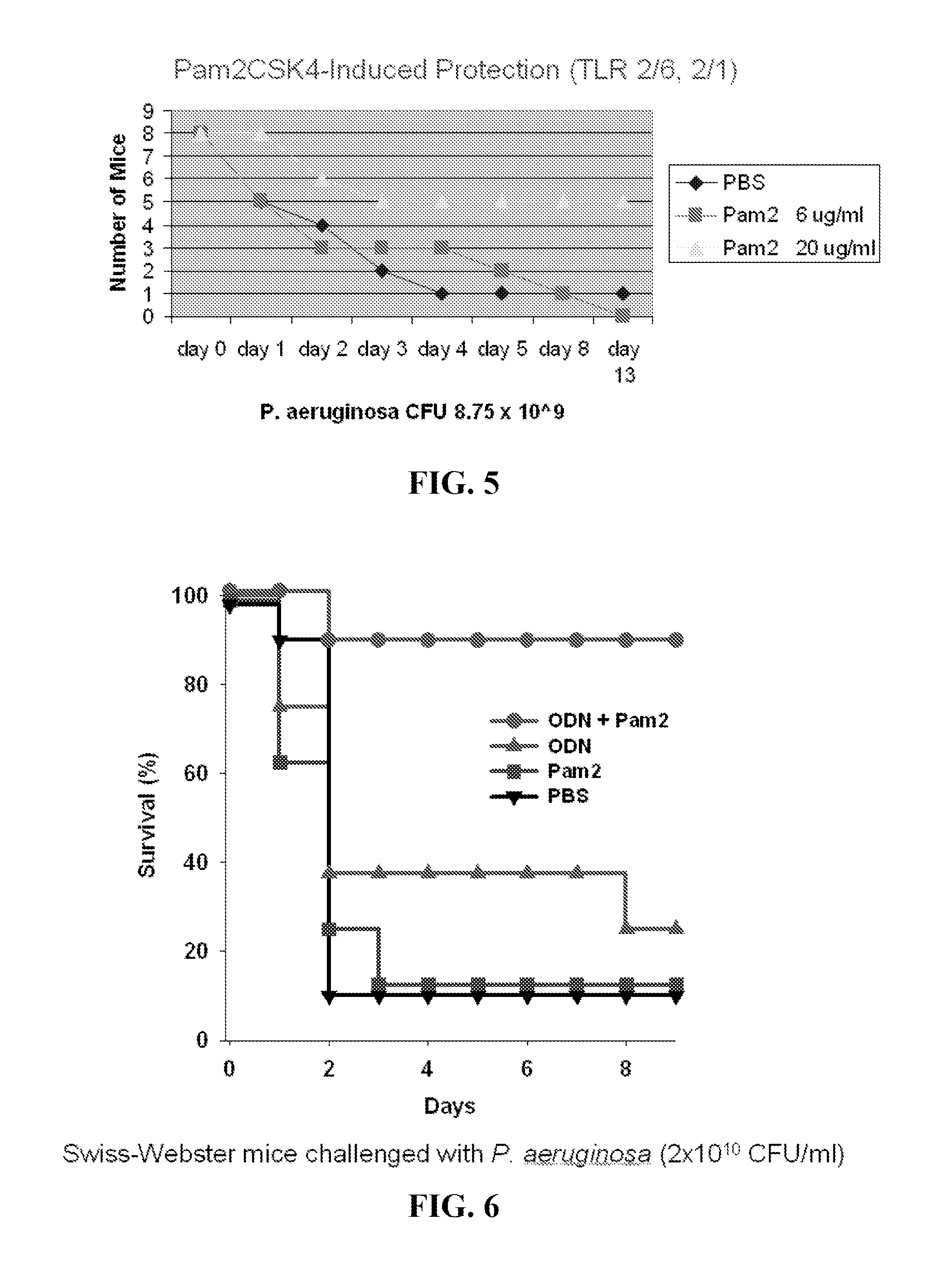
Compositions for stimulation of mammalian innate immune resistance to pathogens
ActiveUS20120082700A1Improve immunityImprove defenseAntibacterial agentsOrganic active ingredientsMicroorganismImmune resistance
Embodiments of the invention are directed to methods of treating, inhibiting or attenuating a microbial infection in an individual who has or is at risk for developing such an infection, comprising the step of administering an effective amount of a TLR9 agonist and a TLR 2 / 6 agonist to the individual.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
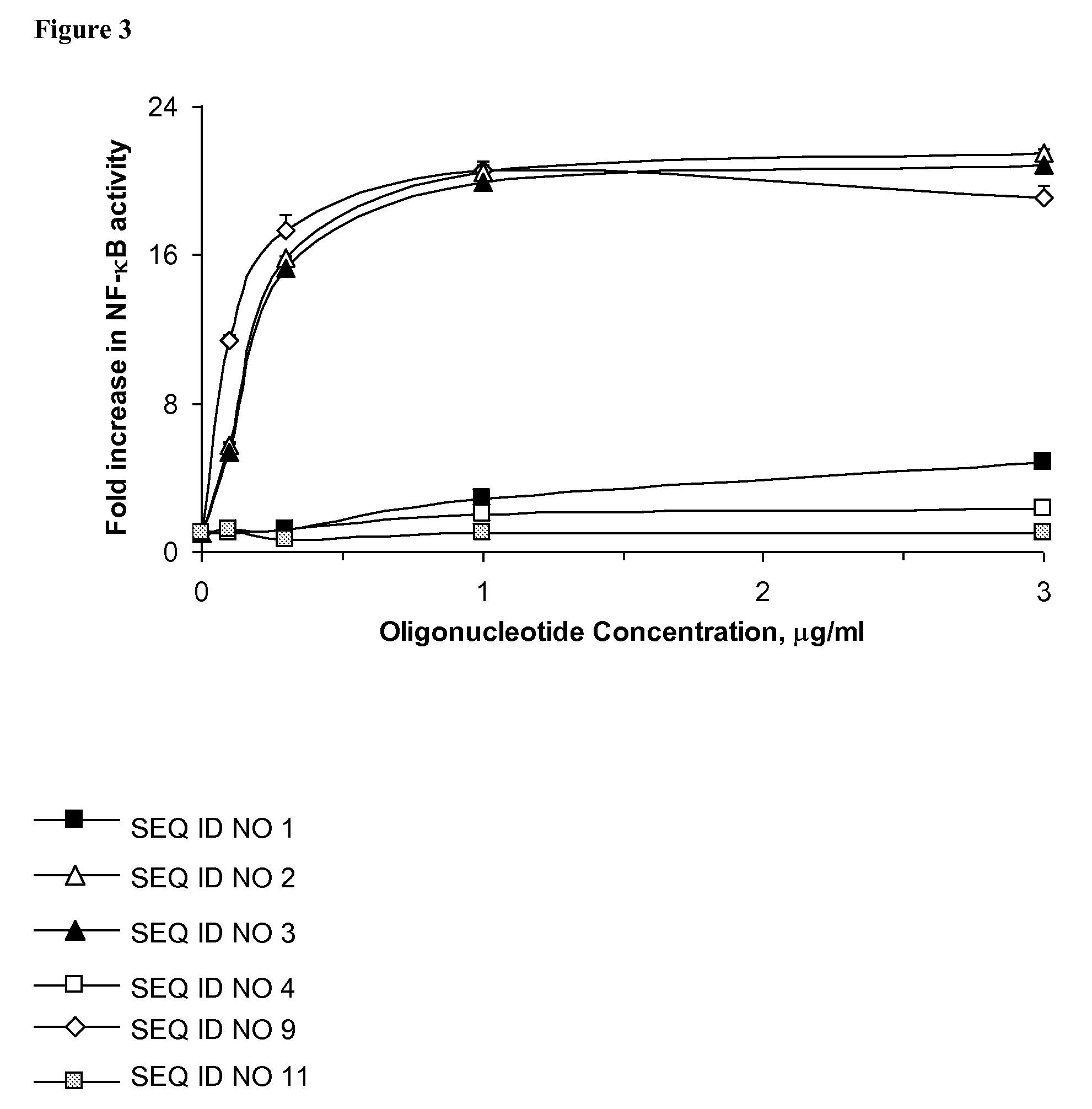
Oligonucleotide and use thereof
InactiveUS8030289B2Sugar derivativesGenetic material ingredientsMultiple organ dysfunction syndromeInterferon production
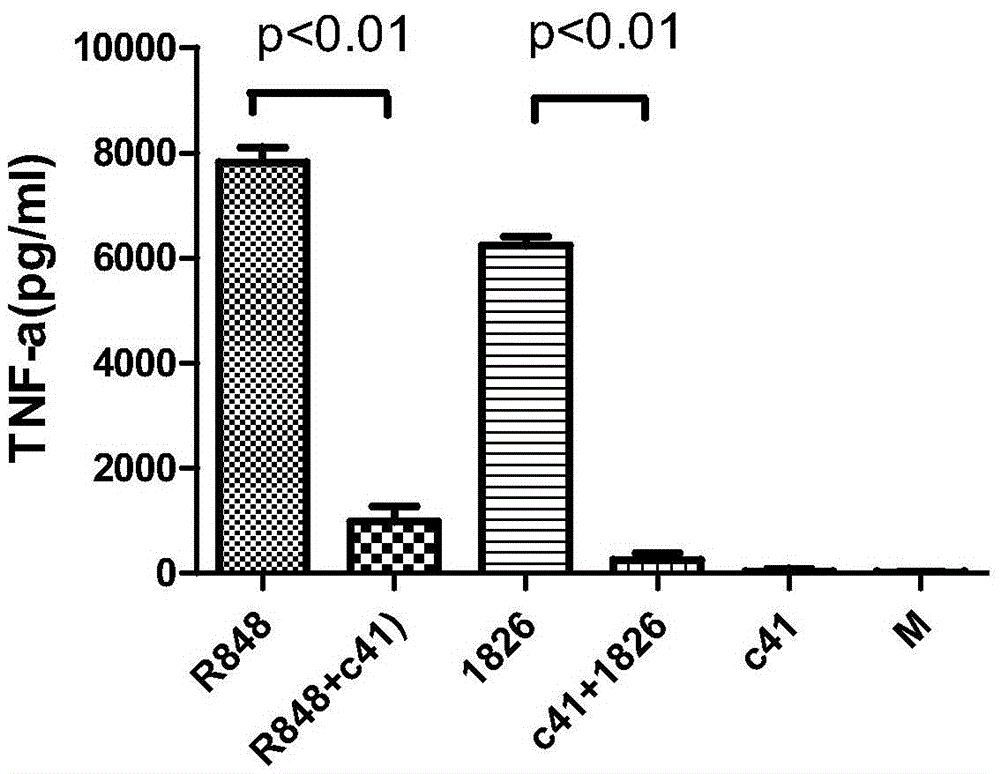
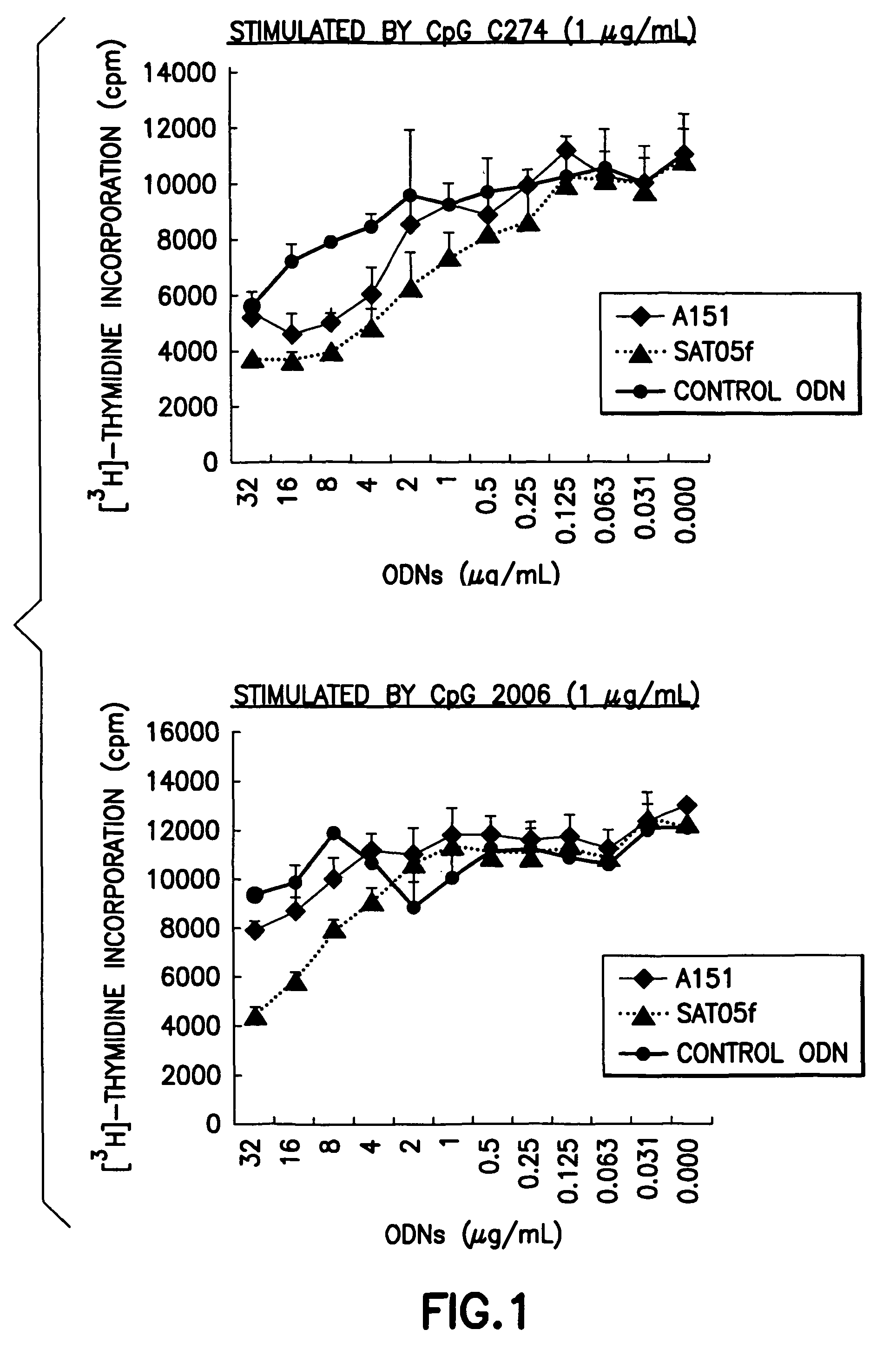
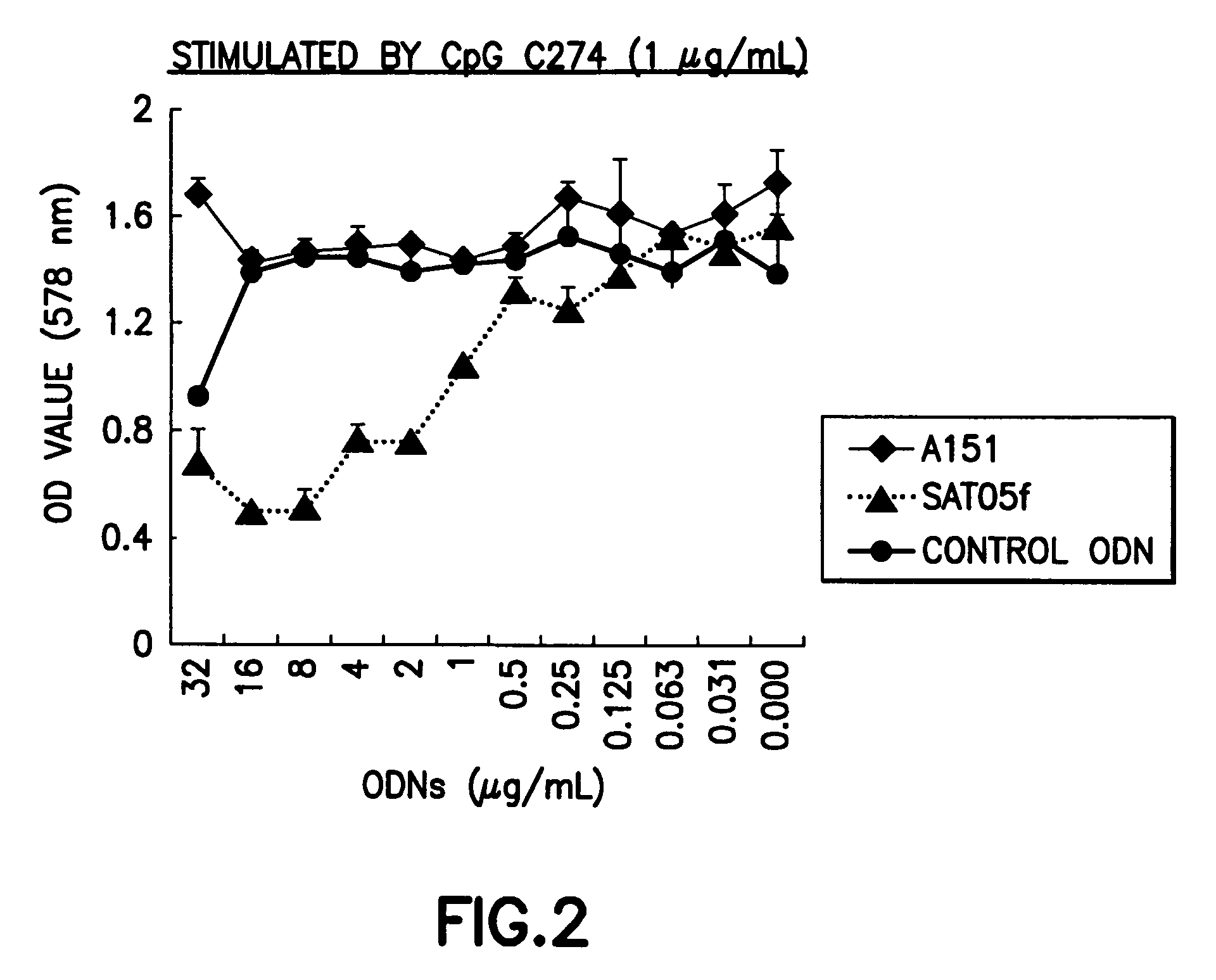
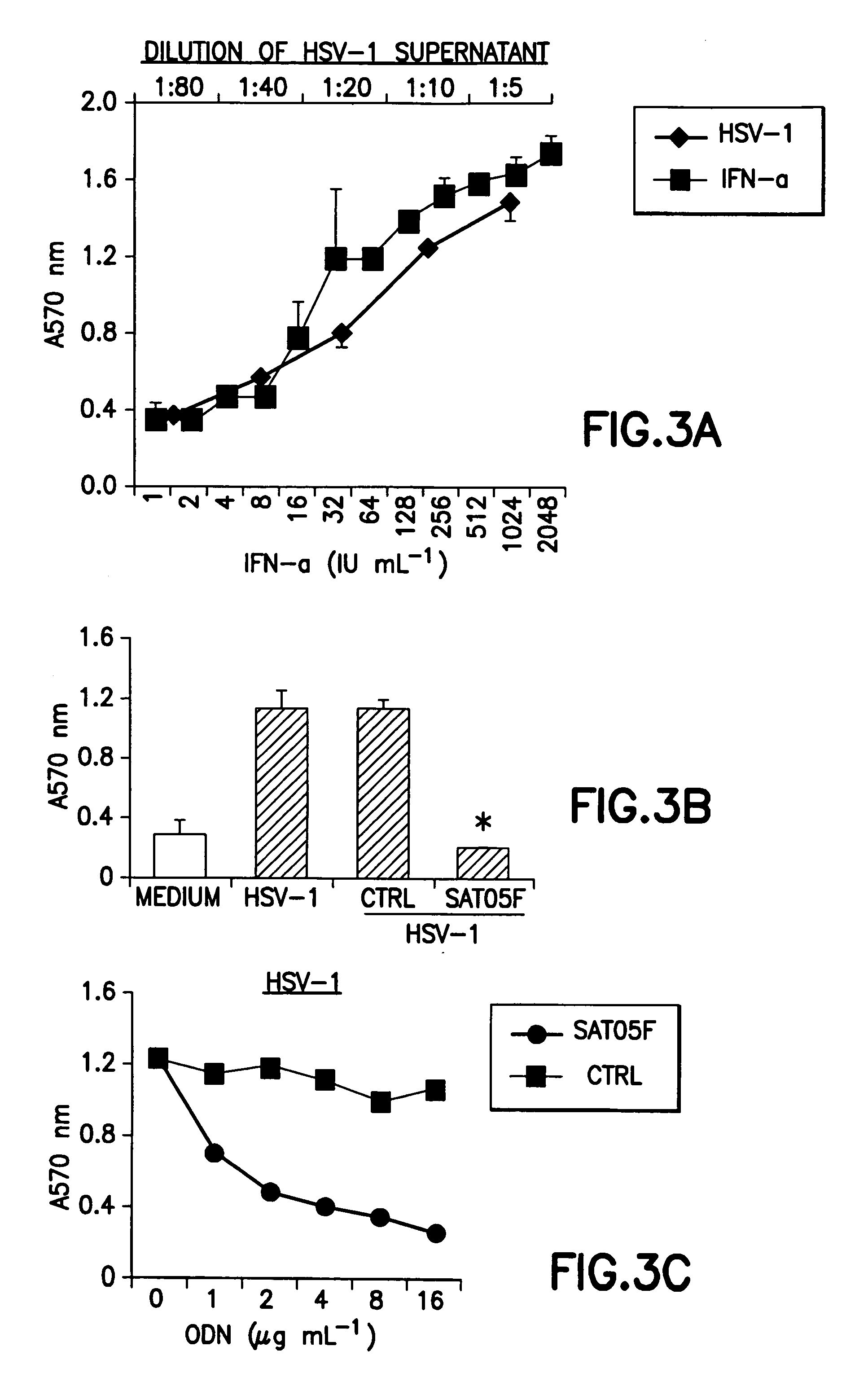
An oligonucleotide with a nucleotide sequence of 5′-cctcctcctcctcctcctcctcct-3′ (SEQ ID NO: 1) inhibits proliferation of human PBMC activated by TLR9 agonist and interferon production from human PBMC induced by TLR9 agonist, HSV-1, flu virus and serum from SLE patients, and rescues the mice from cytokine-mediated lethal shock. This oligonucleotide can be used as a remedy for the treatment of systemic lupus erythematosus (SLE), sepsis, multiple organ dysfunction syndromes and other immune-mediated disorders.
Owner:SBI BIOTECH CO LTD
Pyrrolo[3,2-c]pyridine derivatives as tlr inhibitors
InactiveUS20170008885A1Useful in treatmentOrganic chemistryAntipyreticInflammatory Bowel DiseasesAutoimmune disease
The present invention provides a heterocyclic compound having a TLR7, TLR9, TLR7 / 8, TLR7 / 9 or TLR7 / 8 / 9 inhibitory action, which is useful as an agent for the prophylaxis or treatment of autoimmune diseases, inflammatory diseases and the like, in particular, systemic lupus erythematosus, Sjogren's syndrome, rheumatoid arthritis, psoriasis, inflammatory bowel disease and the like. The present invention is a compound represented by the formula (1): wherein each symbol is as described in the specification, or a salt thereof.
Owner:TAKEDA PHARMA CO LTD
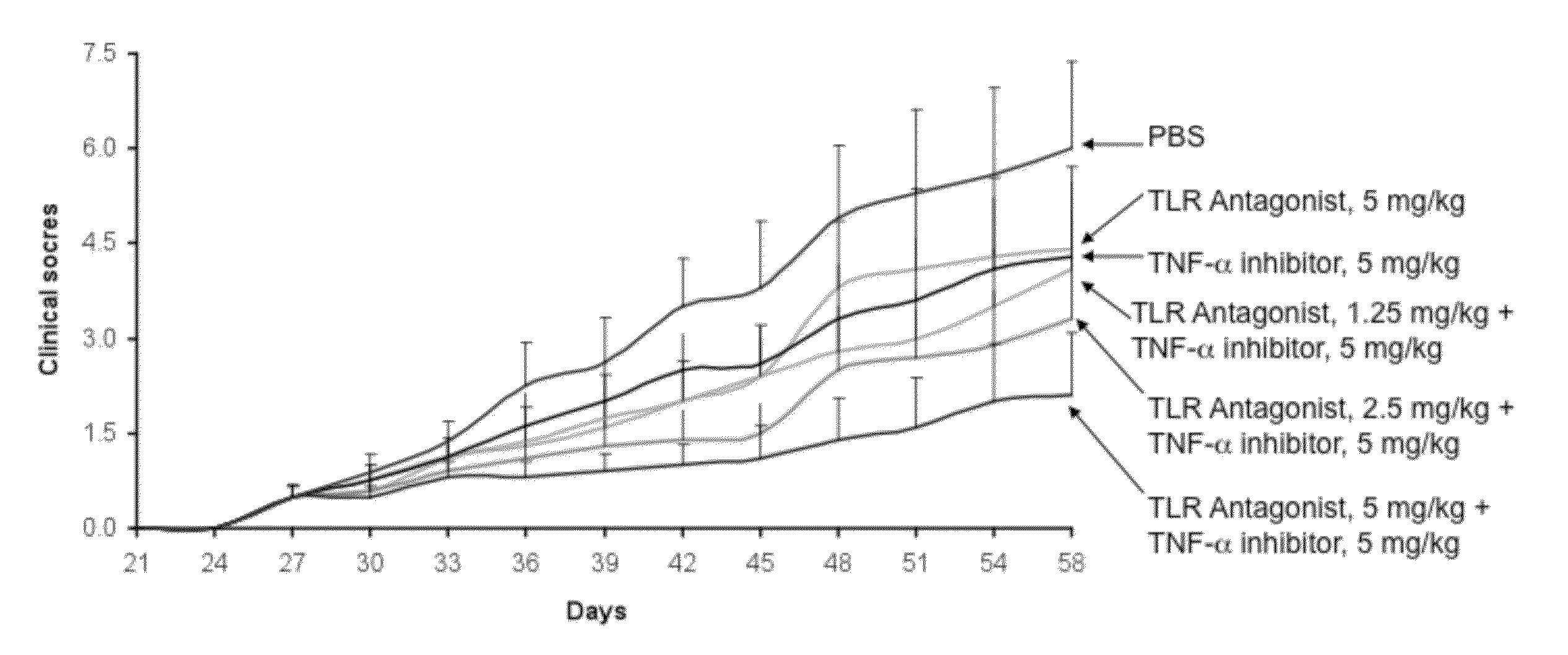
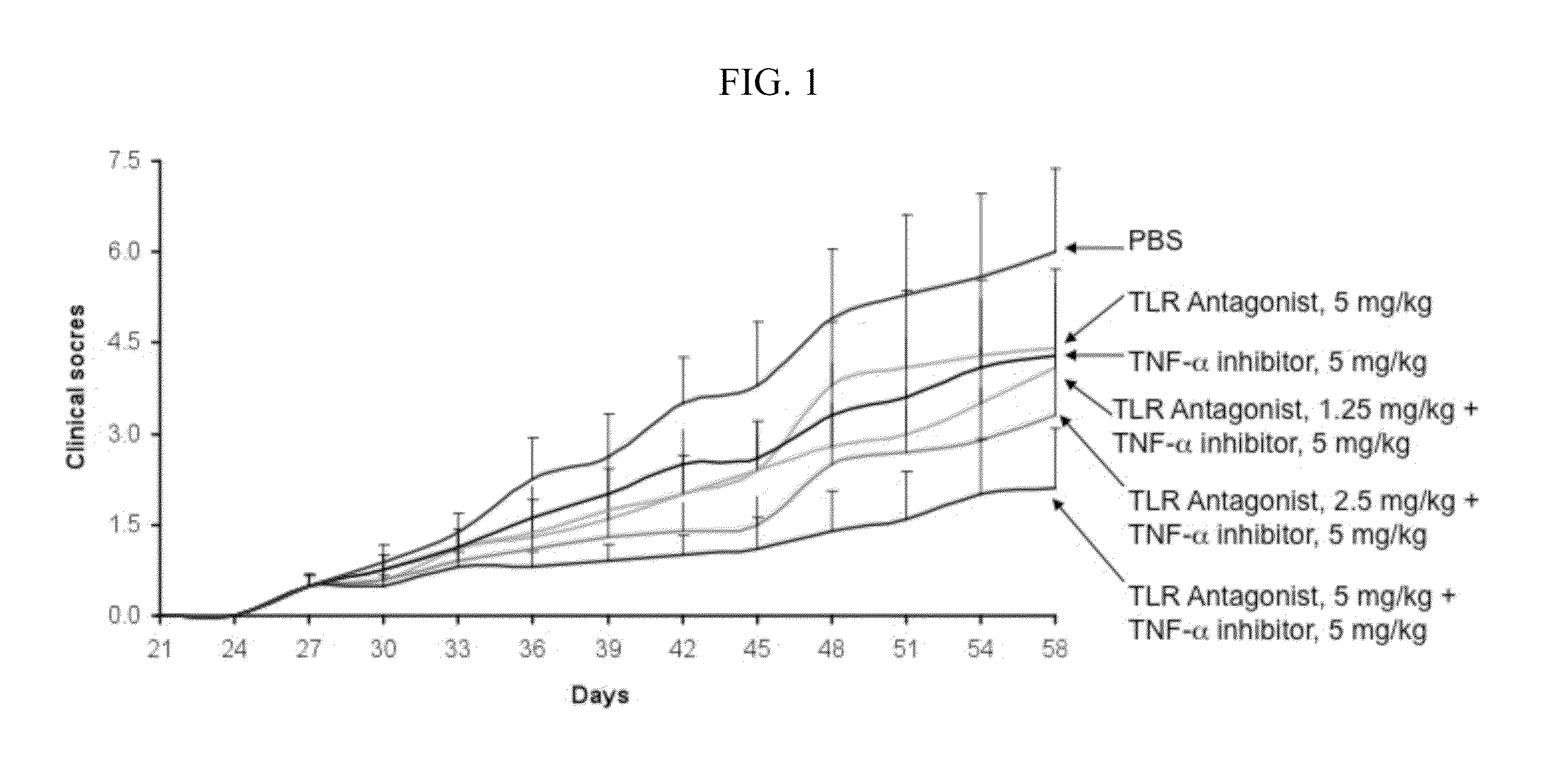
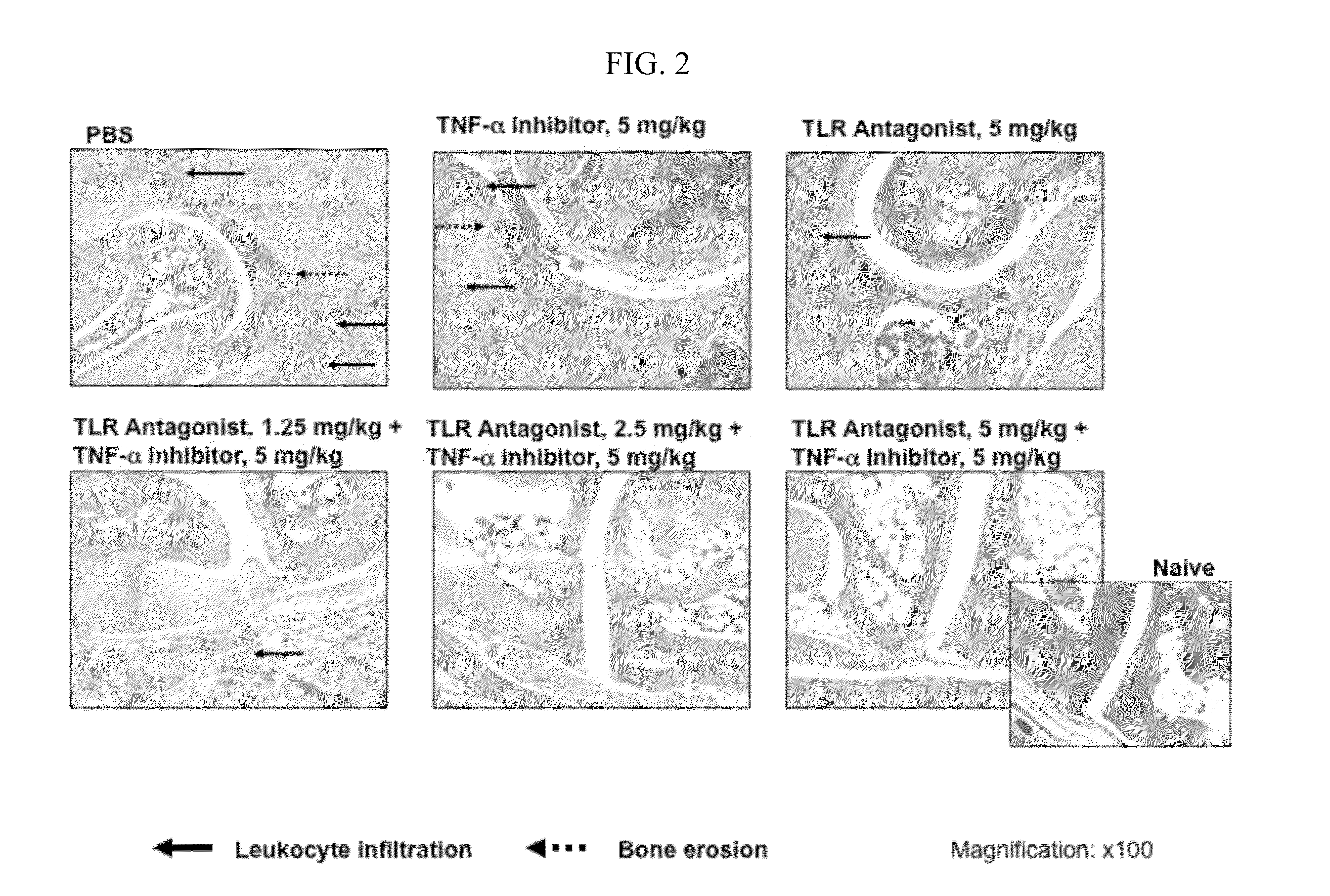
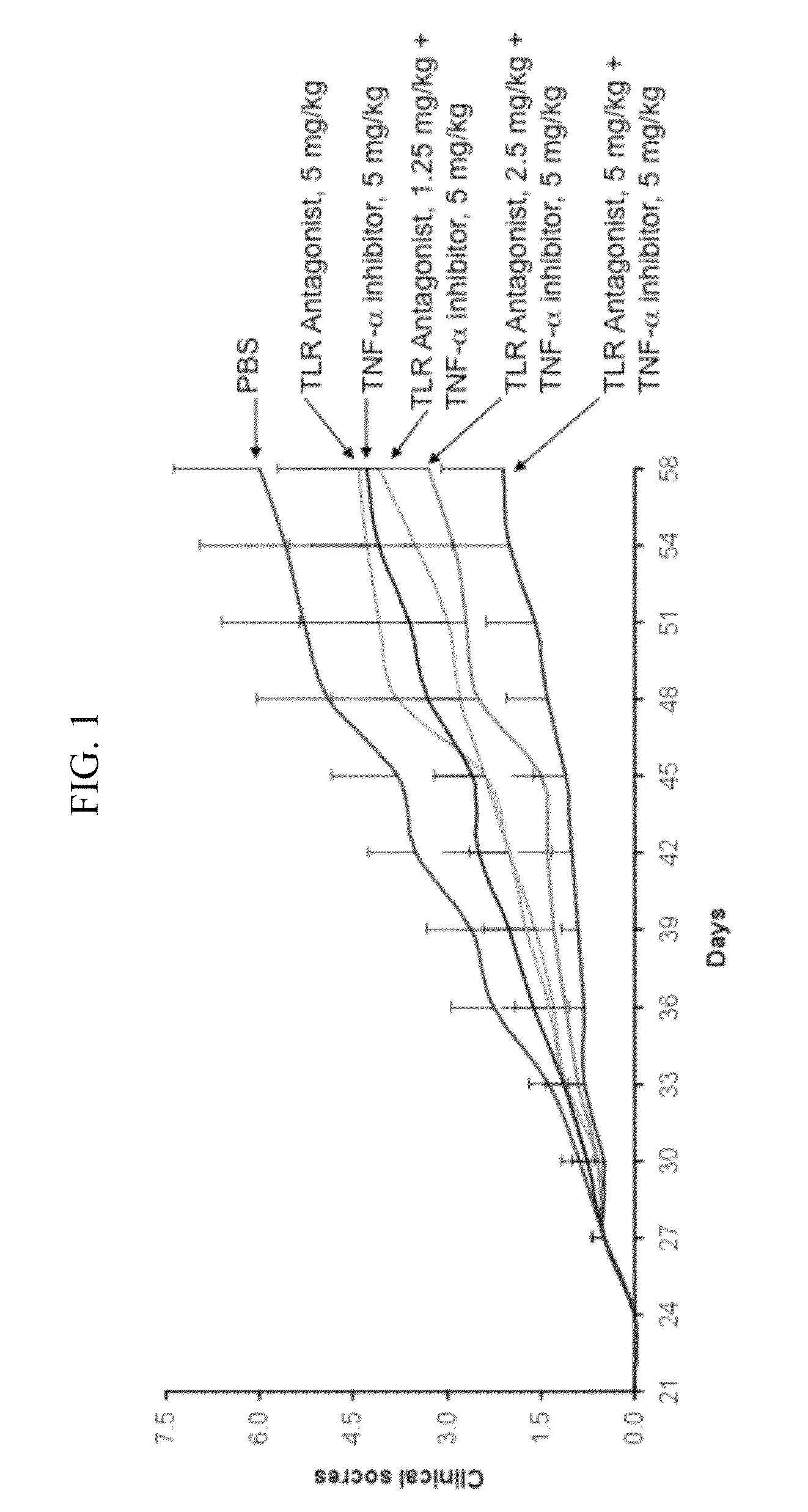
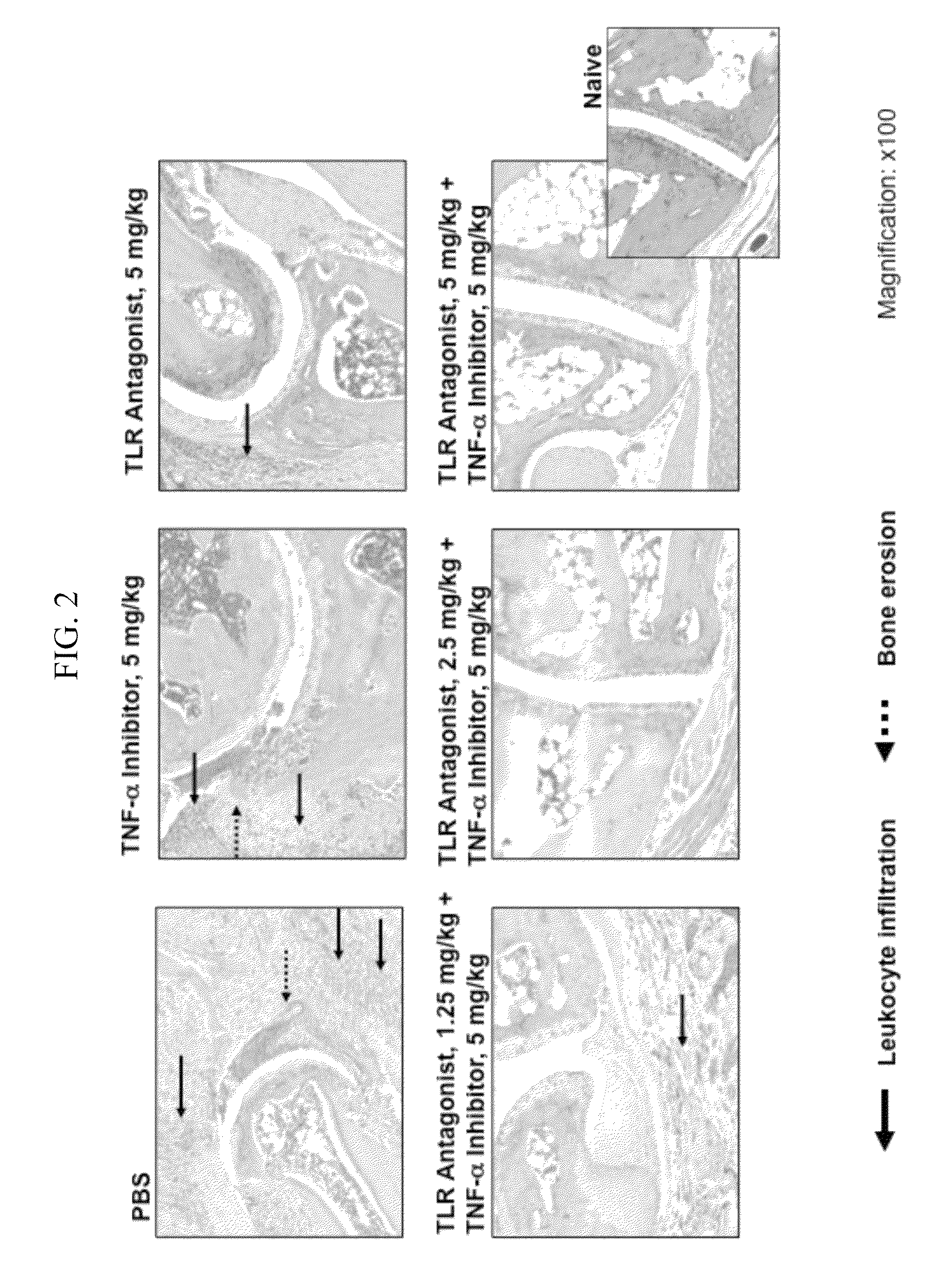
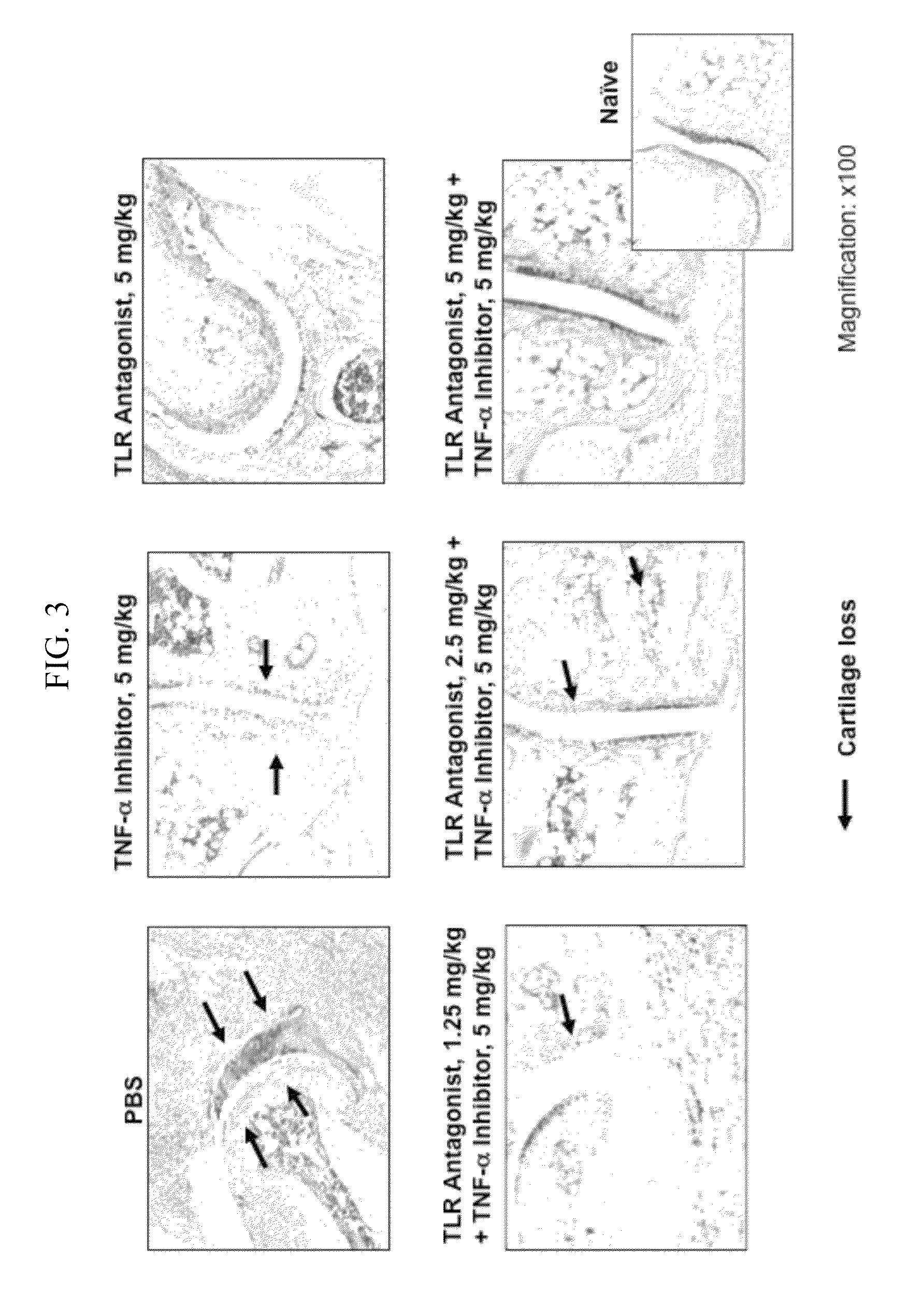
Potentiation of Autoimmune and Inflammatory Disease Treatments by Immune Regulatory Oligonucleotide (IRO) Antagonists of TLR7 and TLR9
ActiveUS20110171209A1Eliminate side effectsHigh activityOrganic active ingredientsPeptide/protein ingredientsDiseaseAutoimmune responses
The invention provides the use of immune regulatory oligonucleotides (IRO) as antagonist of TLRs and potentiators of anti-inflammatory agents that inhibit TNF for the prevention and treatment of inflammatory and autoimmune diseases.
Owner:IDERA PHARMA INC
Potentiation of autoimmune and inflammatory disease treatments by immune regulatory oligonucleotide (IRO) antagonists of TLR7 and TLR9
ActiveUS8987221B2Eliminate side effectsHigh activityOrganic active ingredientsPeptide/protein ingredientsDiseaseAutoimmune responses
Owner:IDERA PHARMA INC
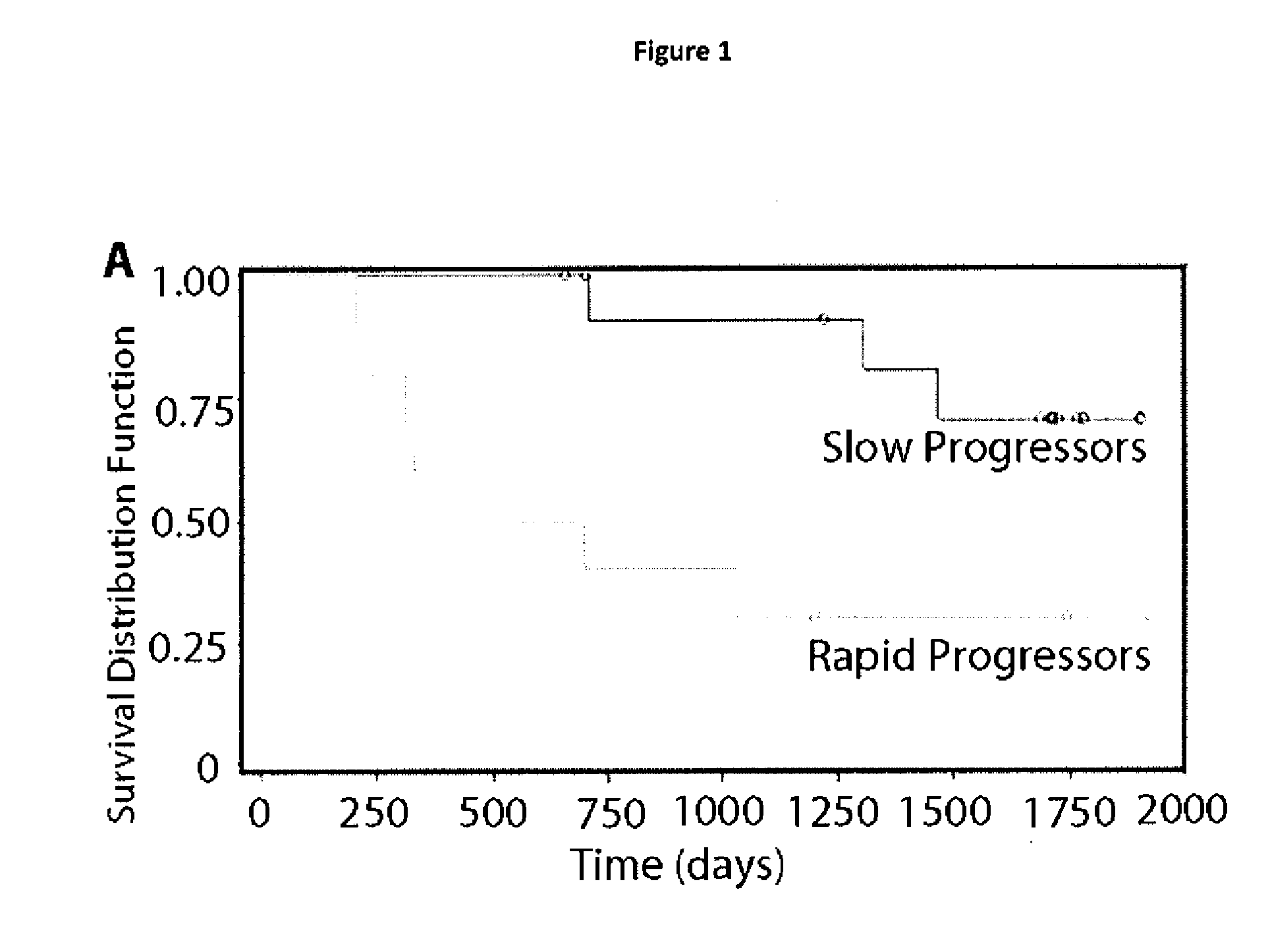
Biomarkers predictive of progression of fibrosis
InactiveUS20120282276A1Fast progressDelay progressOrganic active ingredientsPeptide/protein ingredientsClinical trialFibrosis
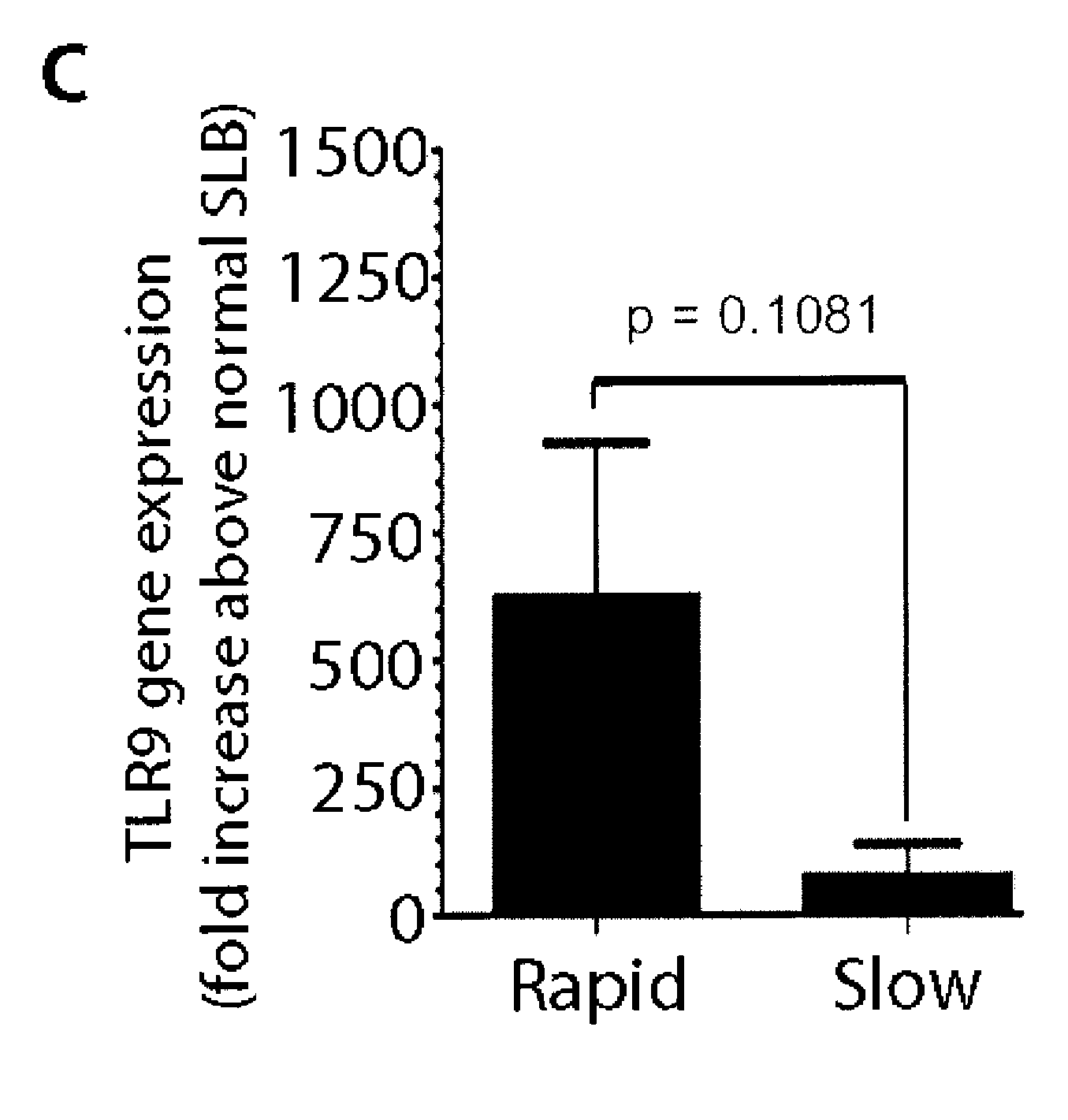
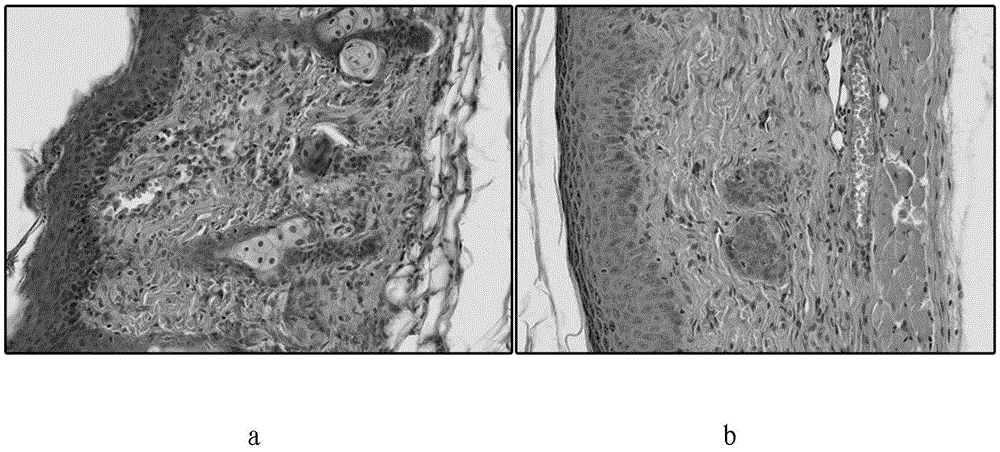
The present invention provides methods and kits for prognosing the progression of fibrosis in a subject having fibrosis, as well as methods for identifying a compound that can slow down the progression of fibrosis in a subject having fibrosis, methods of monitoring the effectiveness of a therapy in reducing the progression of fibrosis in a subject having fibrosis, methods of selecting a subject for participation in a clinical trial for the treatment of fibrosis, and methods for inhibiting progression of fibrosis in a cell or a subject having fibrosis. The methods are based on determining the level of Toll-like recepter 9 (TLR9).
Owner:NOVARTIS AG
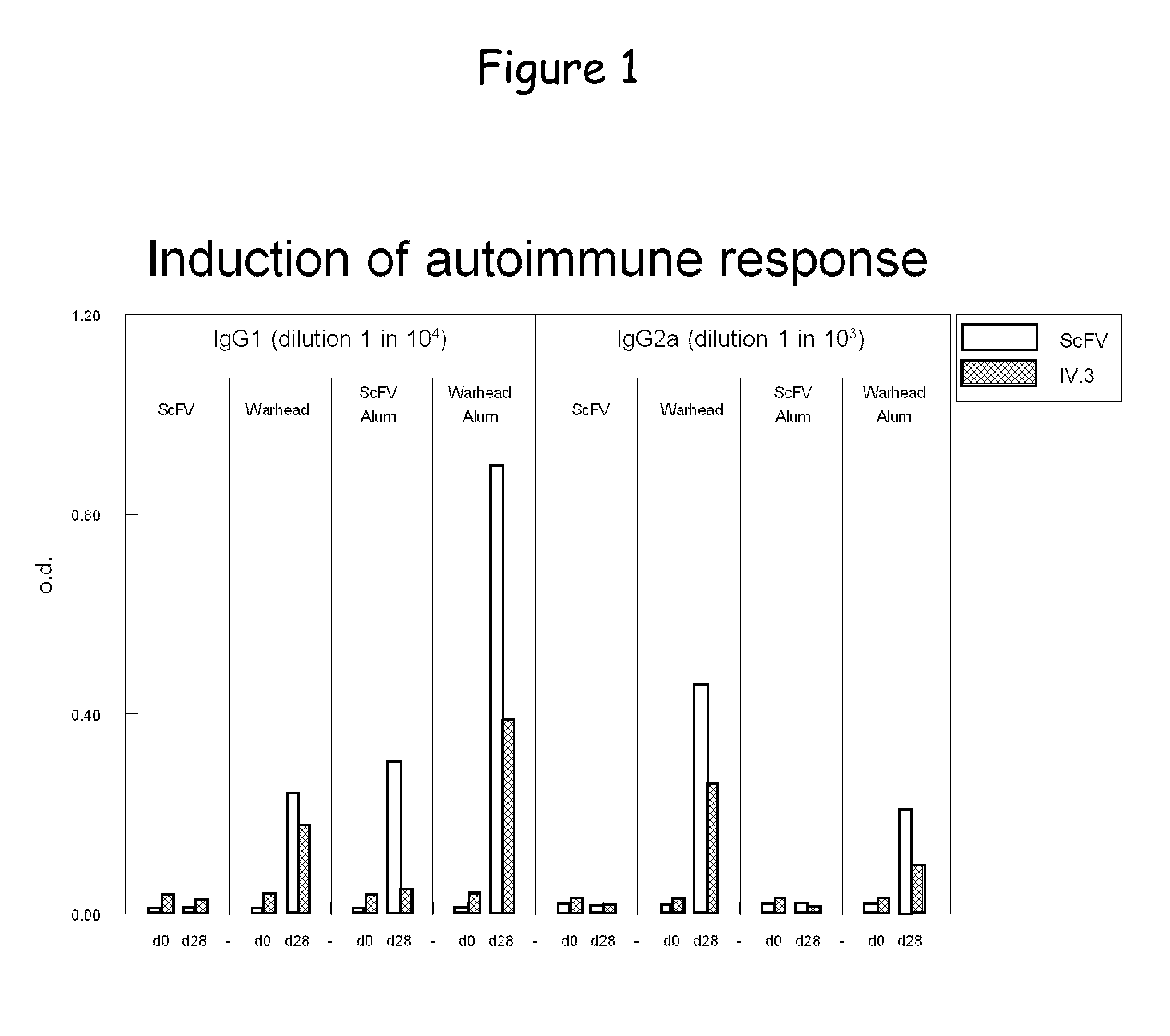
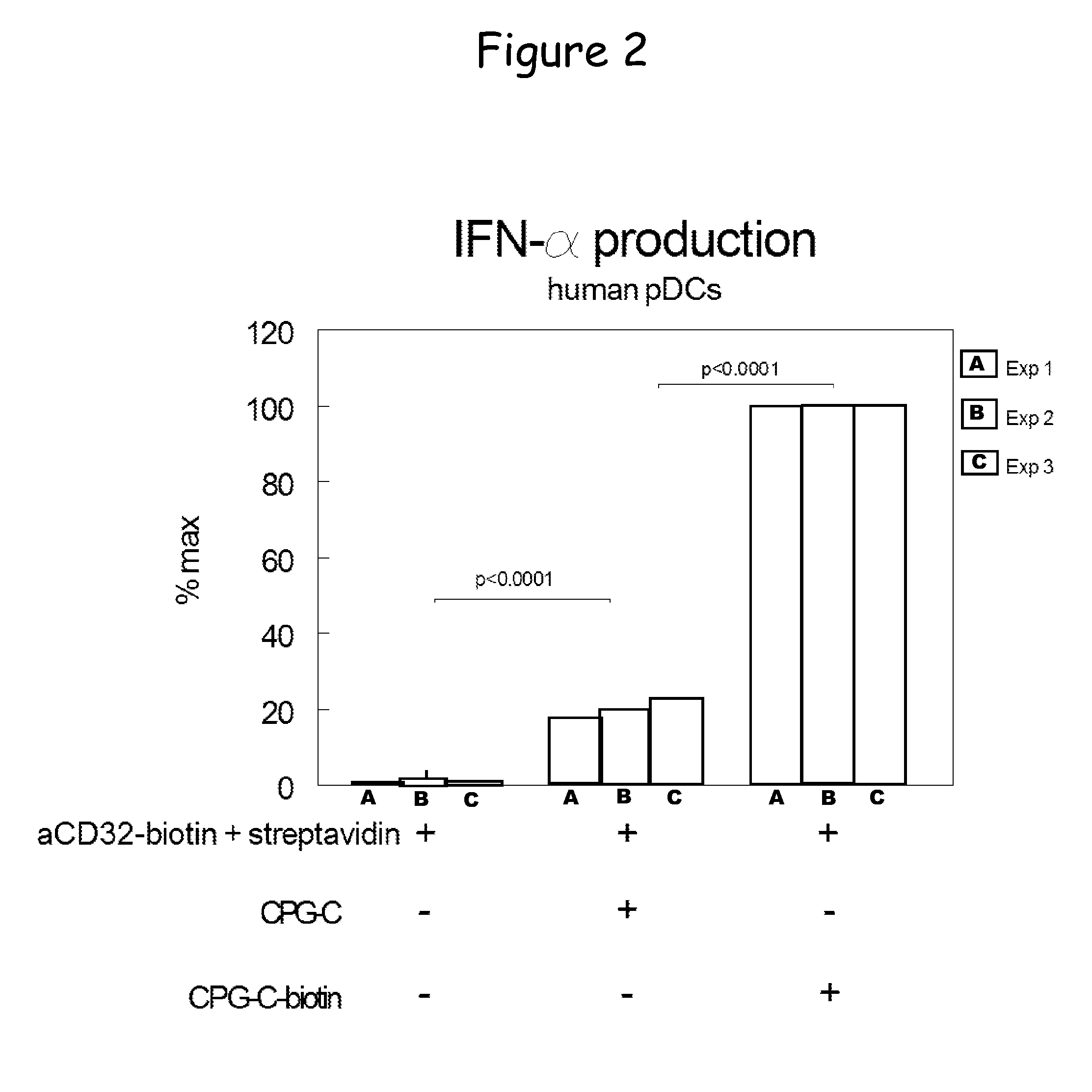
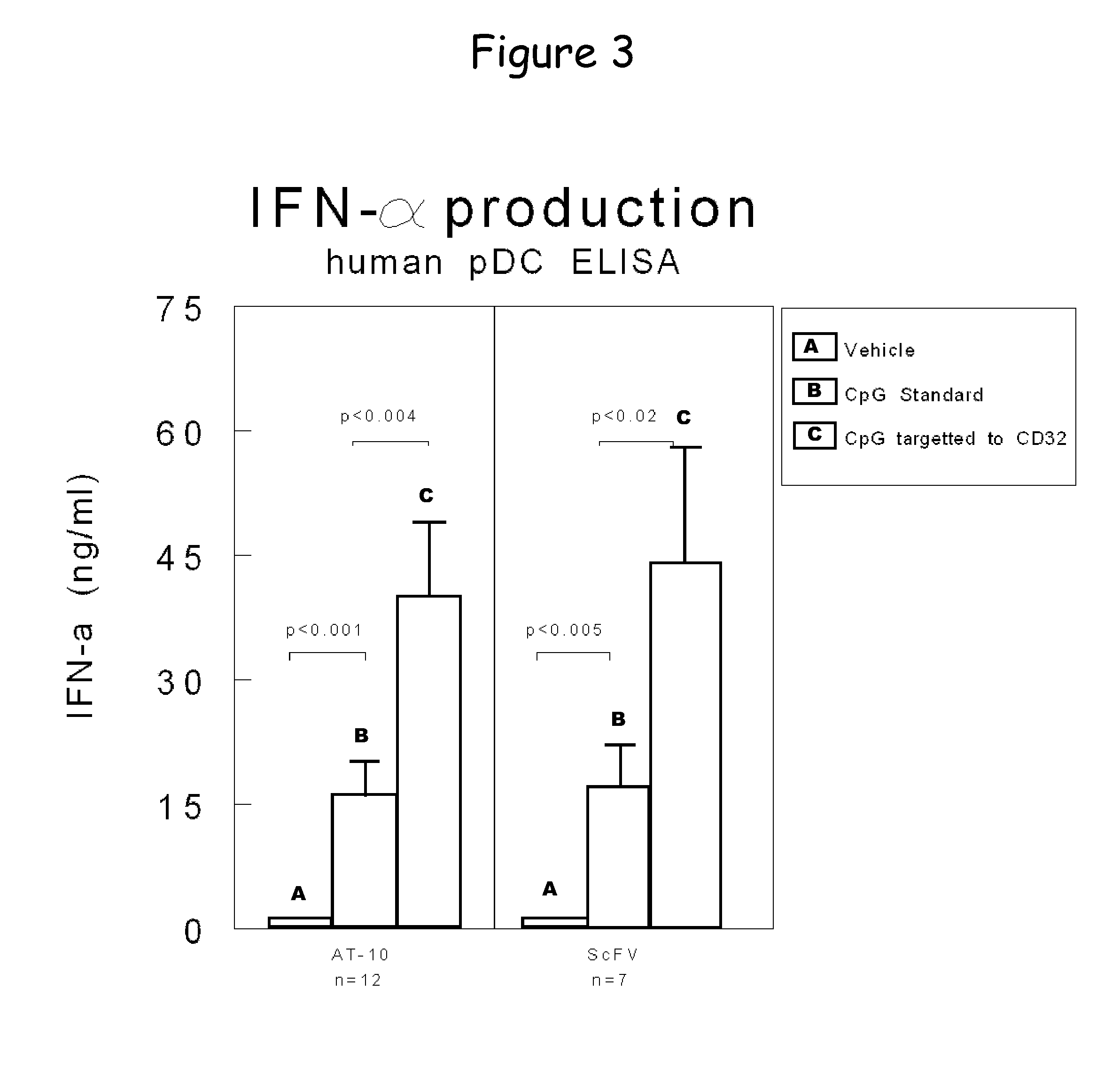
Bispecific molecule binding tlr9 and cd32 and comprising a t cell epitope for treatment of allergies
ActiveUS20130243767A1Prevent allergen presentationPromoting allergen presentationTumor rejection antigen precursorsImmunoglobulins against cell receptors/antigens/surface-determinantsAllergyCD32
Owner:S TARGET THERAPEUTICS
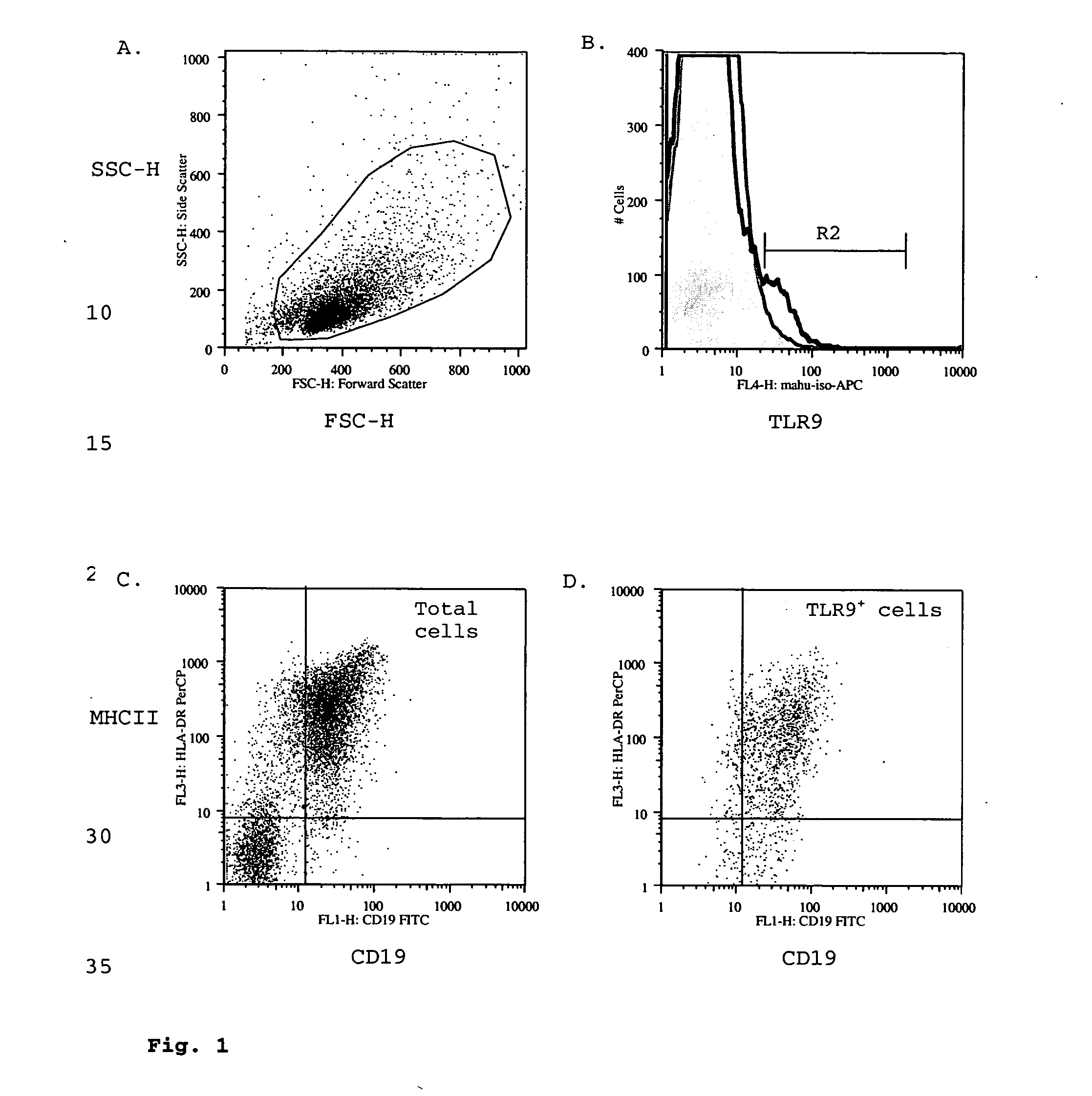
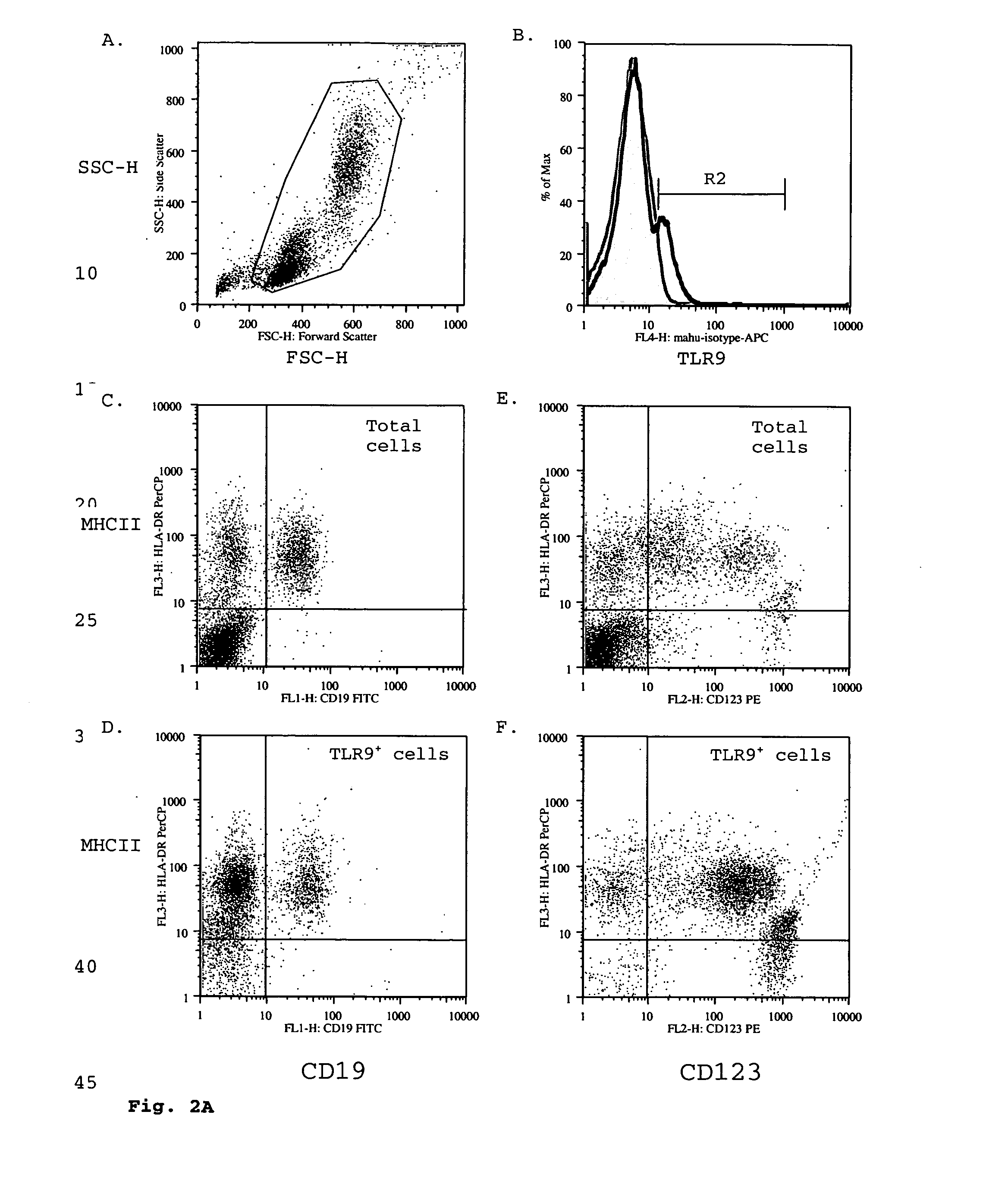
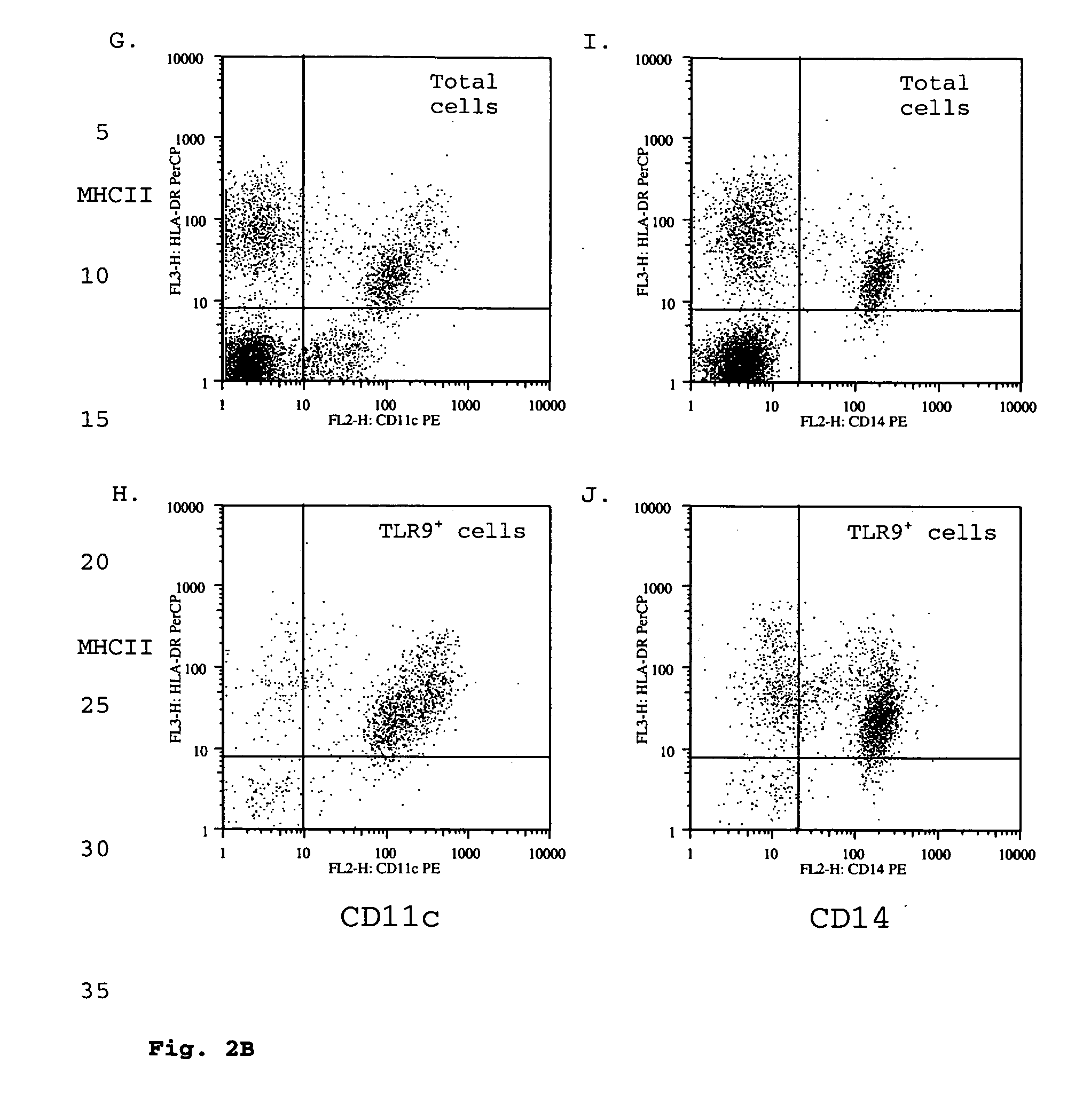
Toll-like receptor 9 effector agents and uses thereof
InactiveUS20050244410A1Genetic material ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsAntibodyToll-Like Receptor 9
Cell surface TLR9 and TLR9 ligand binding agents are disclosed. The binding agents include antibodies and other proteins. The binding agents are useful as therapeutics, diagnostics or research reagents.
Owner:BASSIRI ASHLYN +9
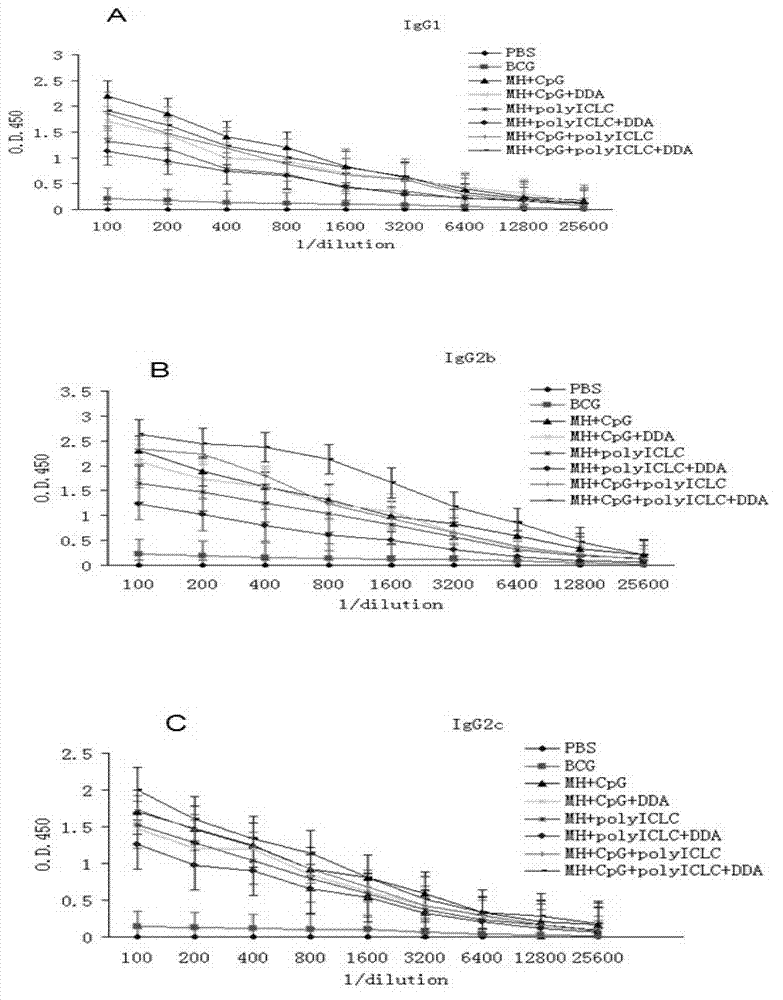
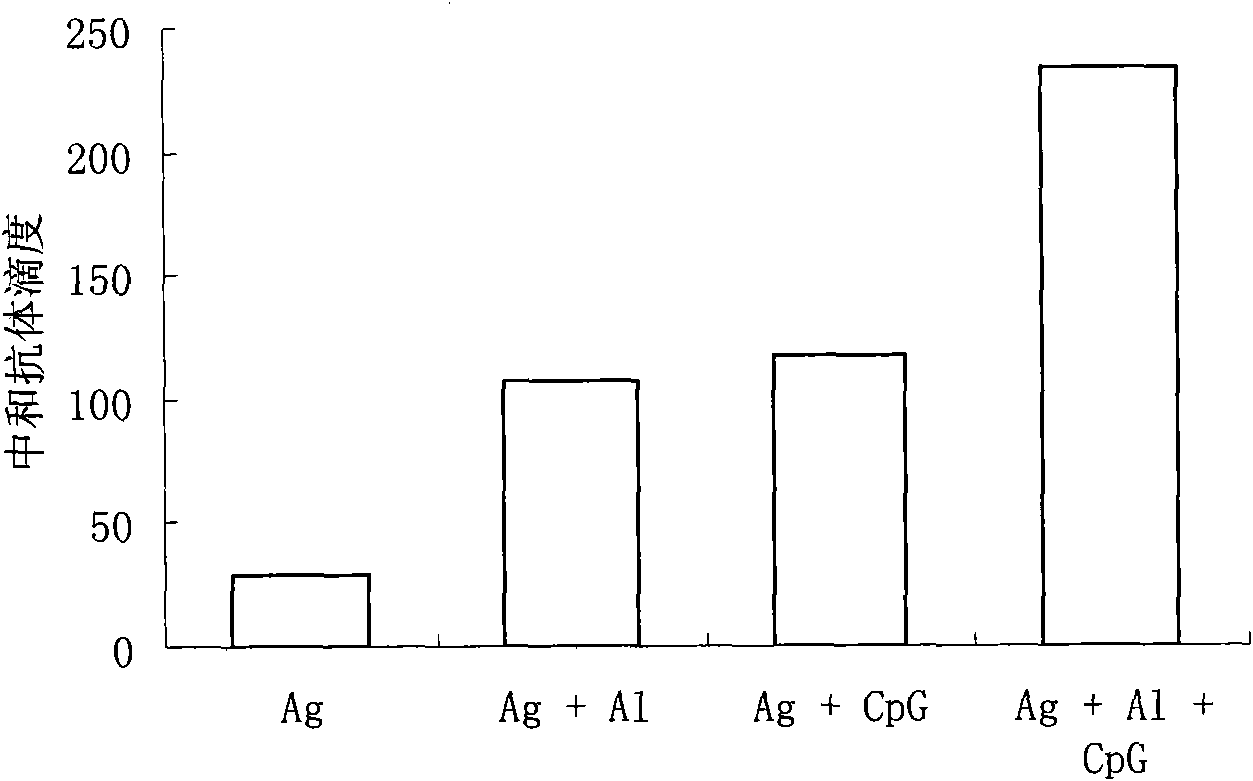
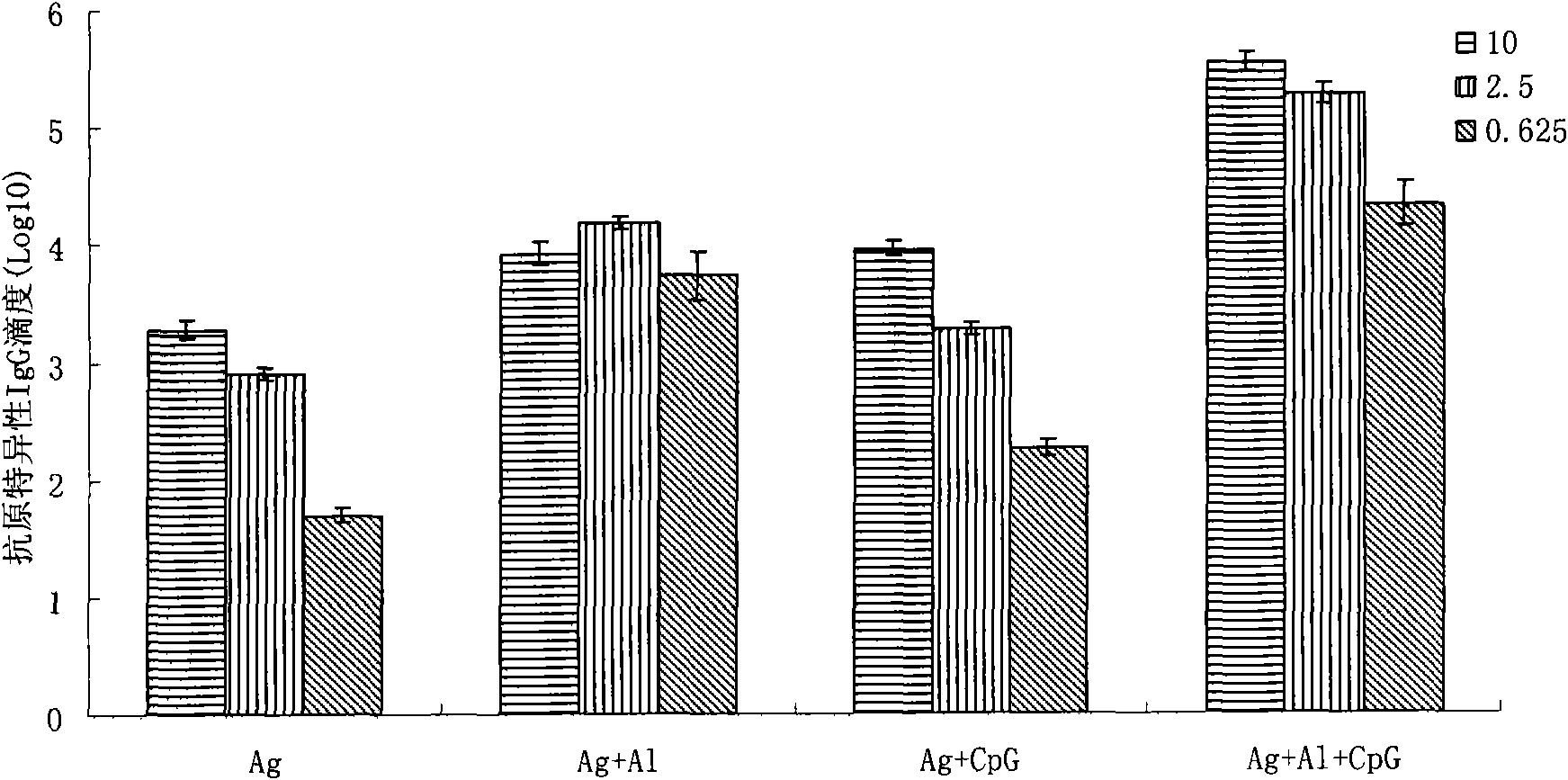
Compound adjuvant for tuberculosis subunit vaccine, tuberculosis subunit vaccine from same and preparation method and application thereof
InactiveCN103203018AImproving immunogenicityHelps induce Th1 type anti-TB immune responseAntibacterial agentsBacterial antigen ingredientsAdjuvantNucleotide
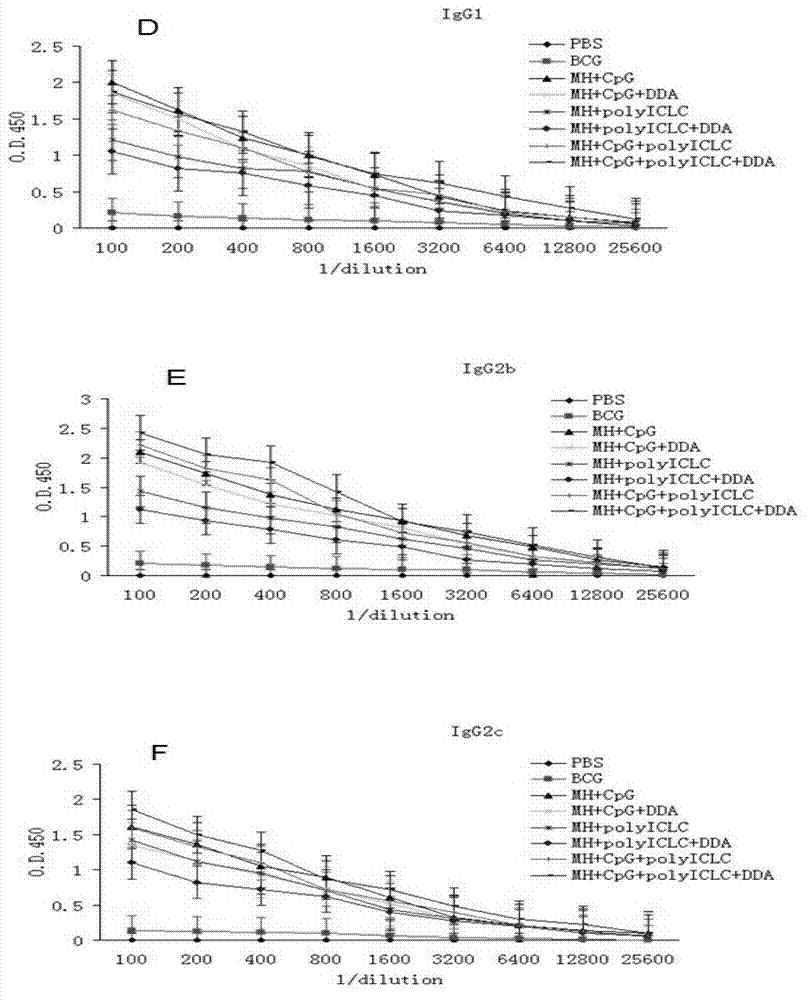
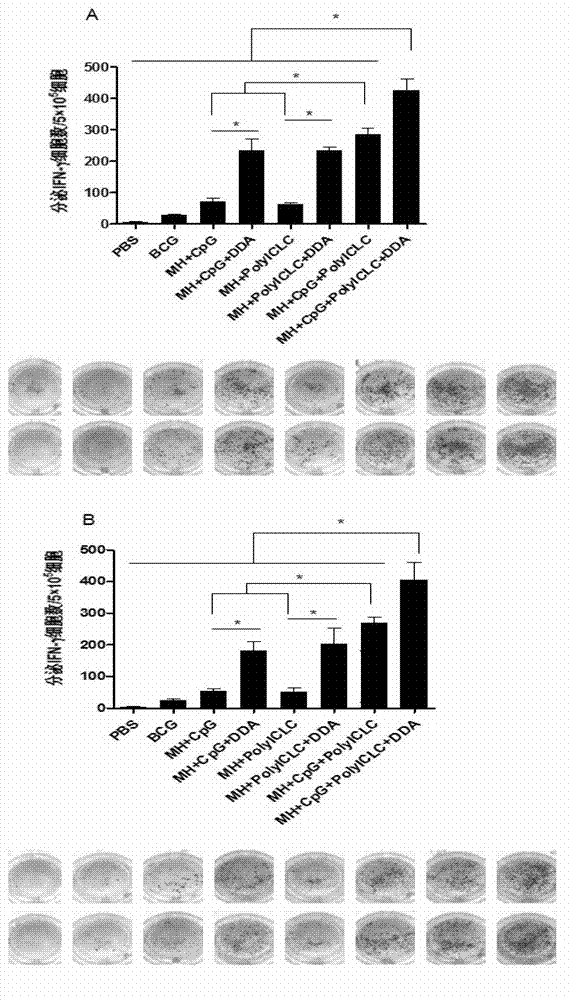
The present invention discloses a compound adjuvant for tuberculosis subunit vaccine, containing cationic liposome dimo-thylidioctyl ammonium bromide (DDA), TLR9 ligand CpG oligodeoxynucleotides 2395 and TLR3 ligand PolyICLC, and also provides the tuberculosis subunit vaccine and preparation method and application thereof. The beneficial effect of the invention is that the compound adjuvant for tuberculosis subunit vaccine provided by the present invention enhances the immunogenicity of the fusion protein of Mycobacterium tuberculosis, contributing to the induction of the tuberculosis fusion protein to Th1-type anti-tuberculosis immune response.
Owner:LANZHOU UNIVERSITY
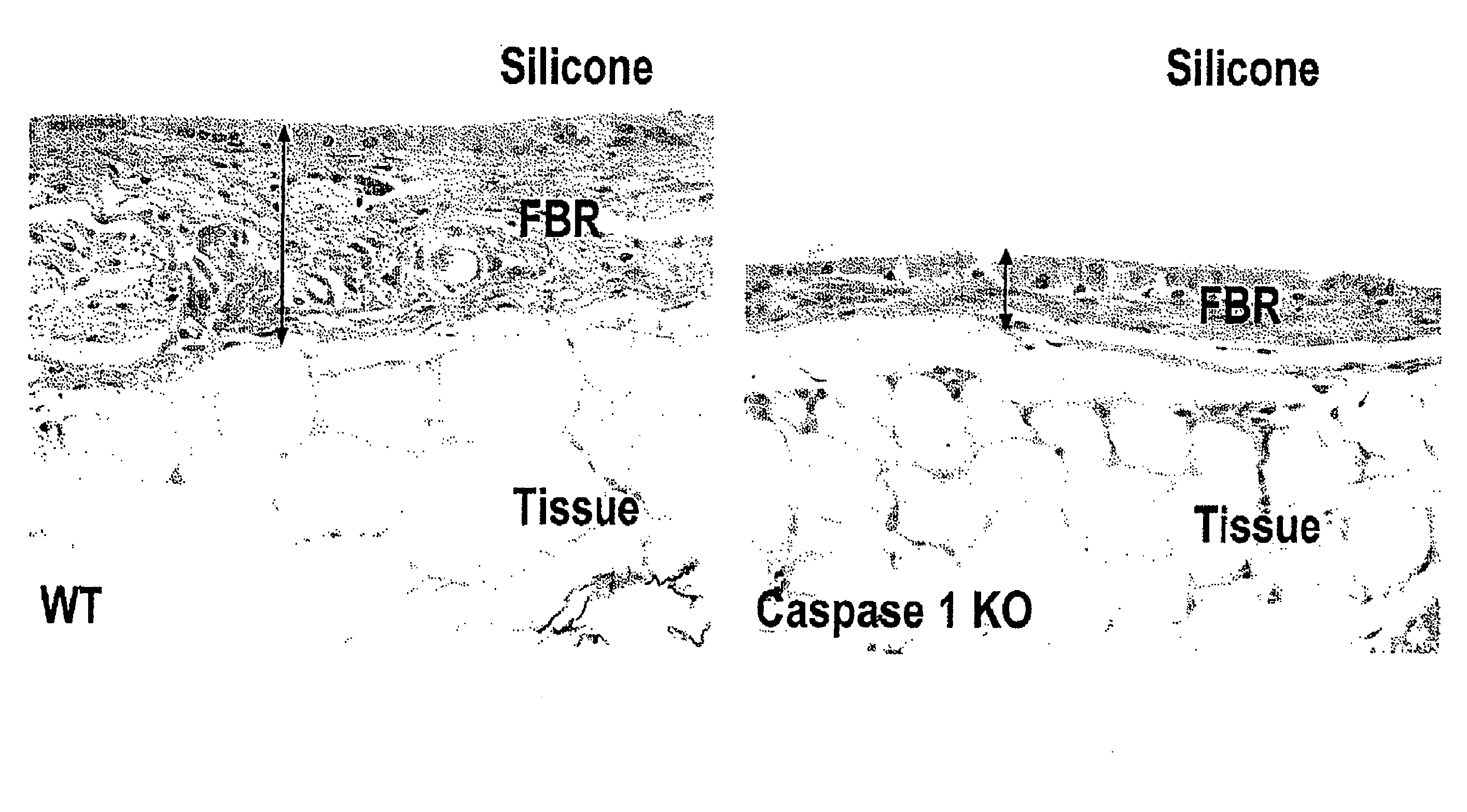
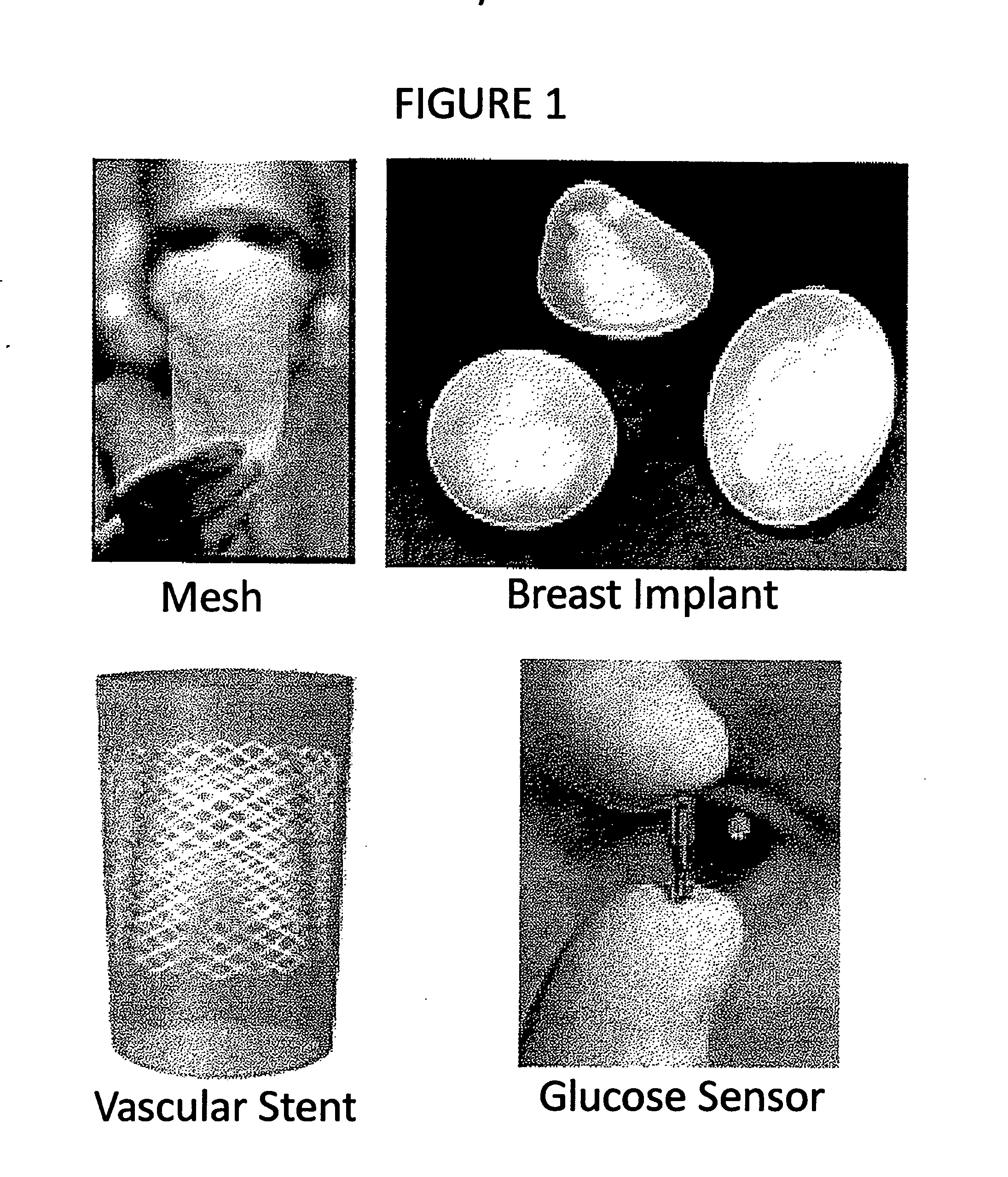
Compositions and methods for inhibiting inflammation from and rejection of biomaterials and other methods
ActiveUS20120225931A1Reduce thicknessReduce the possibilityBiocideAntipyreticHost responseBiological materials
The present invention relates, inter alia, to methods and compositions for regulating the host response to biomaterials, including inhibiting inflammation from and rejection of biomaterials using salicylate compounds and / or TLR7 / TLR9 antagonists as described herein.
Owner:YALE UNIV
HPV (human papillomavirus) peptide/DC (dendritic cell) mixed vaccine and preparation thereof
InactiveCN102008721APromote maturityImprove the level ofViral antigen ingredientsAntiviralsAdjuvantHuman papillomavirus
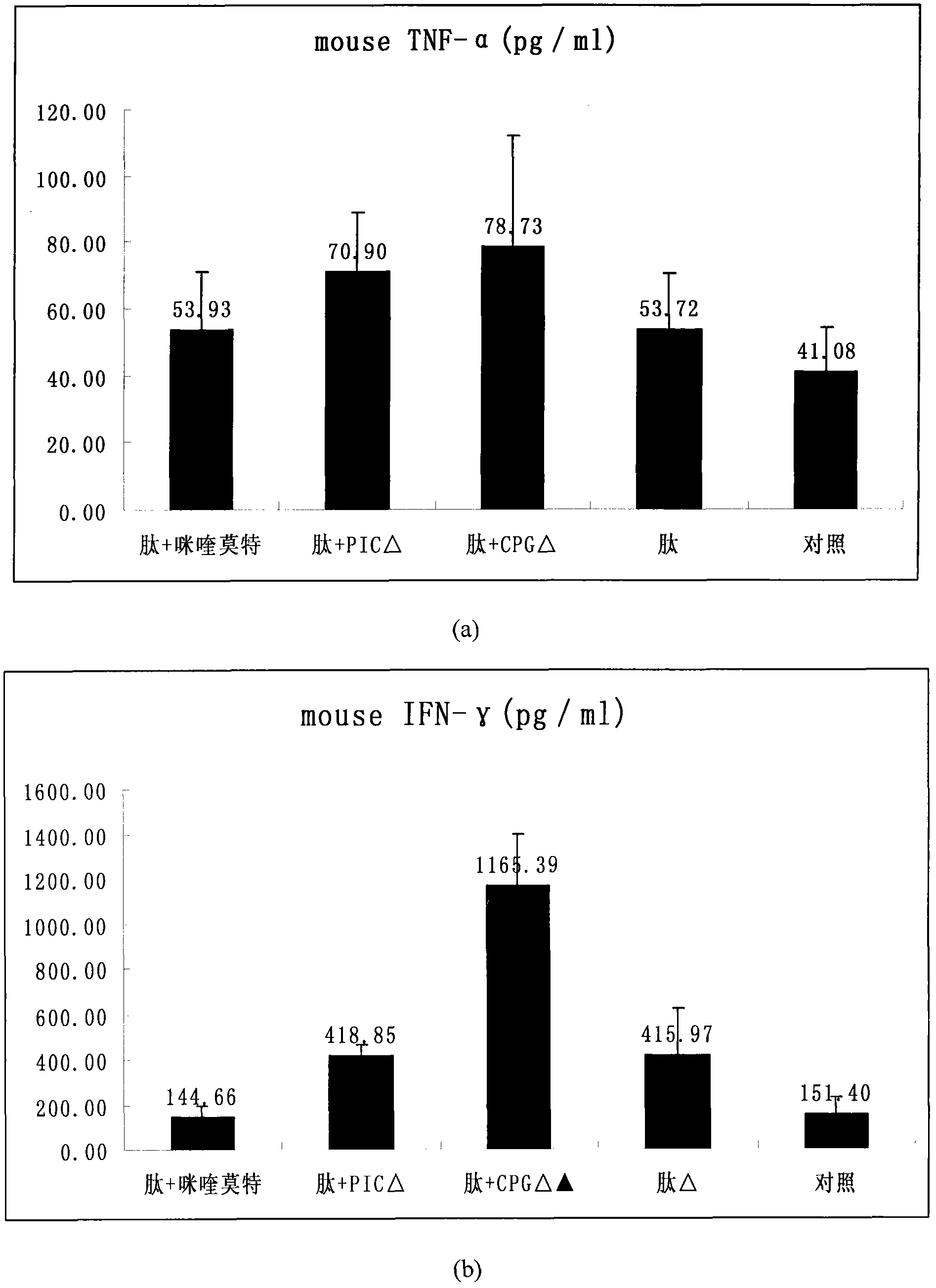
The invention provides an HPV (human papillomavirus) peptide / DC (dendritic cell) mixed vaccine prepared in the presence of a TLR (toll-like receptor) agonist. The mixed vaccine comprises one part of HPV11E77-15, one part of mouse bone marrow-derived dendritic cells which are cultured in vitro and one part of TLR9 agonist as an adjuvant, wherein the dendritic cells are extracted from mouse bone marrow and further cultured in vitro, the co-incubation with one section of HPV11E7CTL (cytotoxic T lymphocyte) epitope peptide with strongest immunogenicity is firstly carried out, the co-incubation with the TLR ligand CpG and the like is further respectively carried out for promoting the maturation of the DC, the degree of maturation can be confirmed by detecting the change of a marker on the surface of the DC after incubation, and the DC vaccine which is simulated to mature can be used for immunizing mice. The TLR ligand and the HPV11E7 peptide are utilized jointly to promote the degree of maturation of the DC, the level of TNF-alpha (tumor necrosis factor-alpha) and IFN-gamma (immunoreactive fibronectin-gamma) of factors of secretory cells of T cells can be particularly improved after combination of the CpG, and the HPV peptide / DC mixed vaccine can be used for preventing and treating genital warts and cervical cancer.
Owner:ZHEJIANG UNIV
Bispecific molecule binding tlr9 and cd32 and comprising a t cell epitope for treatment of allergies
InactiveUS20160362491A1Prevent curingSmall amountSenses disorderHybrid immunoglobulinsMedicineAllergy
Owner:S TARGET THERAPEUTICS
Compositions for stimulation of mammalian innate immune resistance to pathogens
ActiveUS8883174B2Improve immunityImprove defenseAntibacterial agentsOrganic active ingredientsMicroorganismImmune resistance
Embodiments of the invention are directed to methods of treating, inhibiting or attenuating a microbial infection in an individual who has or is at risk for developing such an infection, comprising the step of administering an effective amount of a TLR9 agonist and a TLR 2 / 6 agonist to the individual.
Owner:BOARD OF RGT THE UNIV OF TEXAS SYST
Medicine for treating psoriasis
InactiveCN105597079AClearly targetedClear locationPeptide/protein ingredientsPharmaceutical delivery mechanismCuticlePhosphate
The invention relates to a medicine for treating psoriasis. The medicine is prepared from a phosphorothioate modified molecule preparation and phosphate buffer, wherein the phosphorothioate modified molecule preparation is used as the active ingredient and has the sequence shown in SEQ ID NO:1. The medicine can resist activation of TLR7 / 8 and TLR9, restrain release of proinflammatory factors such as TNF and IL, and achieve an inflammation restraining effect. By means of a surface microneedle system, micropores penetrating through a cuticle are established without causing the pain feeling, the medicine smoothly enters affected parts through inunction and is diffused in corium layers, activation of aggregated immune cells is prevented, and the pathological injury by psoriasis can be remarkably relieved.
Owner:THE FIRST AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIVERSITY OF PLA
Regulation of Toll-Like Receptors on Stem Cells
InactiveUS20070087408A1Reduce expressionReduce functionBiocideTissue cultureProgenitorHematopoietic cell
The discovery of Toll-like receptors (TLRs) on the surface of hematopoietic cells provides new methods for the stimulation and differentiation of various classes of progenitor cells. TLR2 and TLR4 agonists (natural ligands, mimetics, antibodies) are particularly useful in these methods. The cells can be isolated and used for various purpose including tissue regeneration and grafting. In contrast, antagonists of TLRs can be used to protect cells from various insults such as chemo- and radiotherapy, acute and chronic infection, and transplantation by inhibiting activation and differentiation. TLR2, TLR4 and TLR9 pathway antagonists (soluble TLR, mimetics, antibodies) are particularly useful in these methods. Cells can be isolated and used for various purposes including transplantation.
Owner:OKLAHOMA MEDICAL RES FOUND
Engineered viral vector reduces induction of inflammatory and immune responses
InactiveUS20190316151A1Low immunogenicityReduce inflammationOrganic active ingredientsAntipyreticViral vectorImmunity response
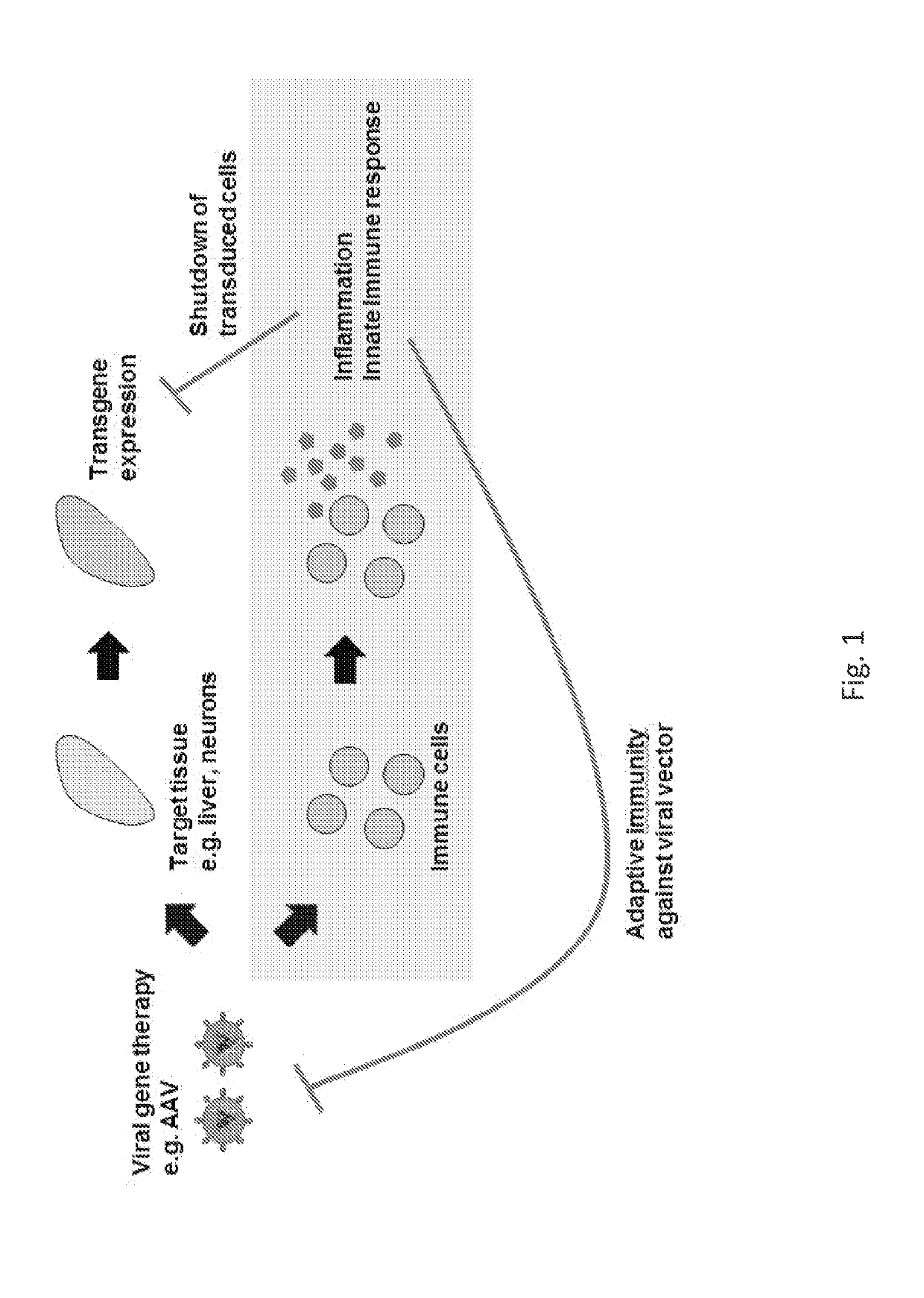
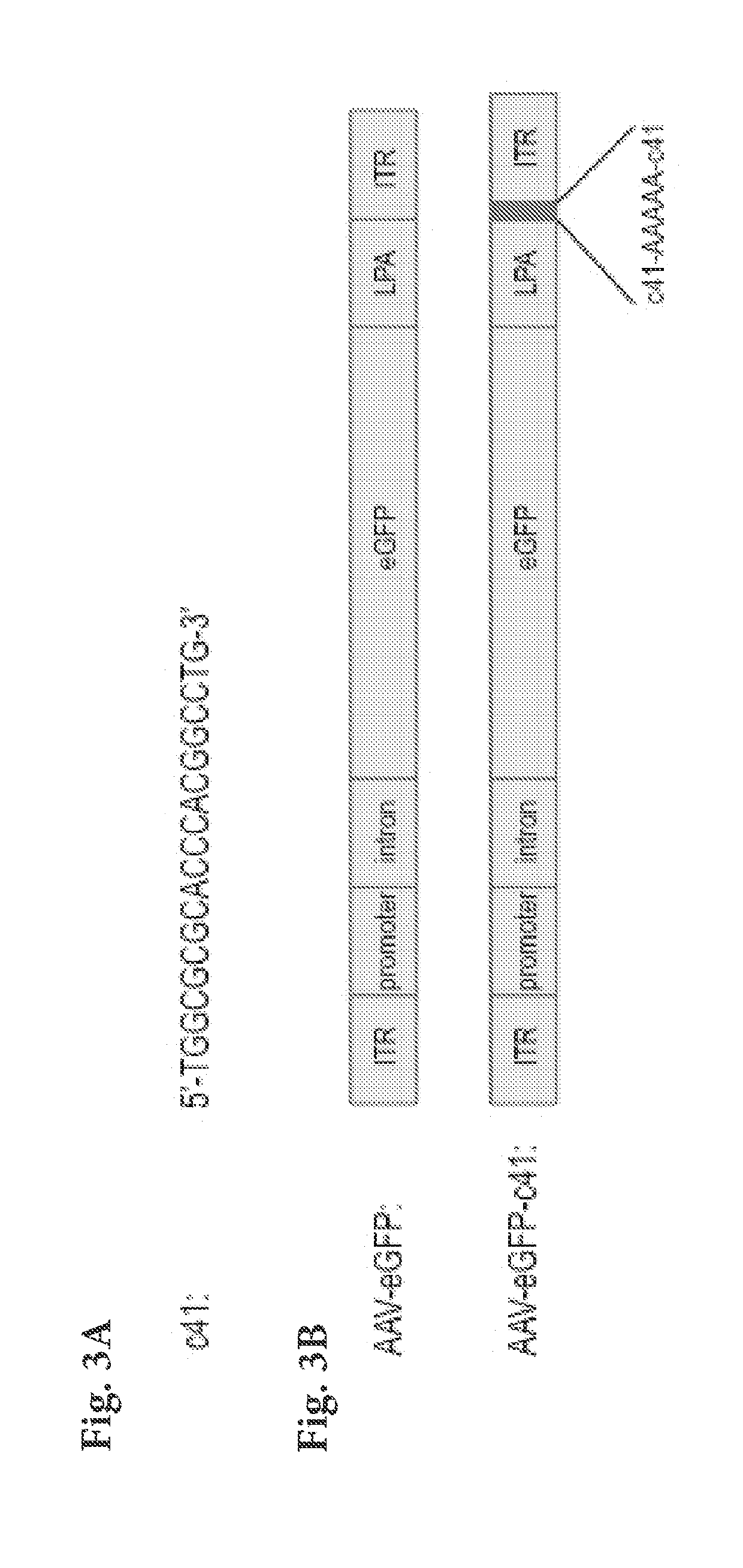
Modified viral genomes are able to reduce induction of inflammatory and immune anti-viral responses. This manifests itself in reduced NF-kB activity, increased viral transduction rates, and increased expression of transgenes. Viral genomes are modified by incorporating one or more oligonucleotide sequences which are able to bind to TLR9 but not induce activation of it. The oligonucleotide sequences may be synthetic, bacterial, human, or from any other source.
Owner:PRESIDENT & FELLOWS OF HARVARD COLLEGE
Meningococcus vaccine
InactiveCN101559225AImprove immunityLow costAntibacterial agentsCarrier-bound antigen/hapten ingredientsSpecific immunityCarrier protein
The invention relates to a meningococcus vaccine comprising a chemical conjugate of meningococcus capsular polysaccharide and carrier protein, and a first adjuvant, wherein the first adjuvant is oligodeoxynucleotide comprising at least one non-methylation CpG dinucleotide, and the length of the oligodeoxynucleotide is 15-35 ribonucleotides. The adjuvant used for increasing the immunization effect of the meningococcus vaccine is a novel human vaccine adjuvant with favorable potential application prospect, and the chemical essence of the adjuvant is the oligodeoxynucleotide which is synthesized manually and contains the CpG dinucleotide. By a receptor TLR9 activated immune system on immune cells, the adjuvant reinforces specific immune response aiming at vaccine antigens. The immunization effect of a vaccine can be increased by adding the vaccine adjuvant into the vaccine, so that the dosage of the vaccine antigen can be reduced, the immunization injection times can be reduced and the vaccine cost can be reduced.
Owner:NAT VACCINE & SERUM INST
RNA interference sequence inhibiting mouse TLR9 expression and application thereof
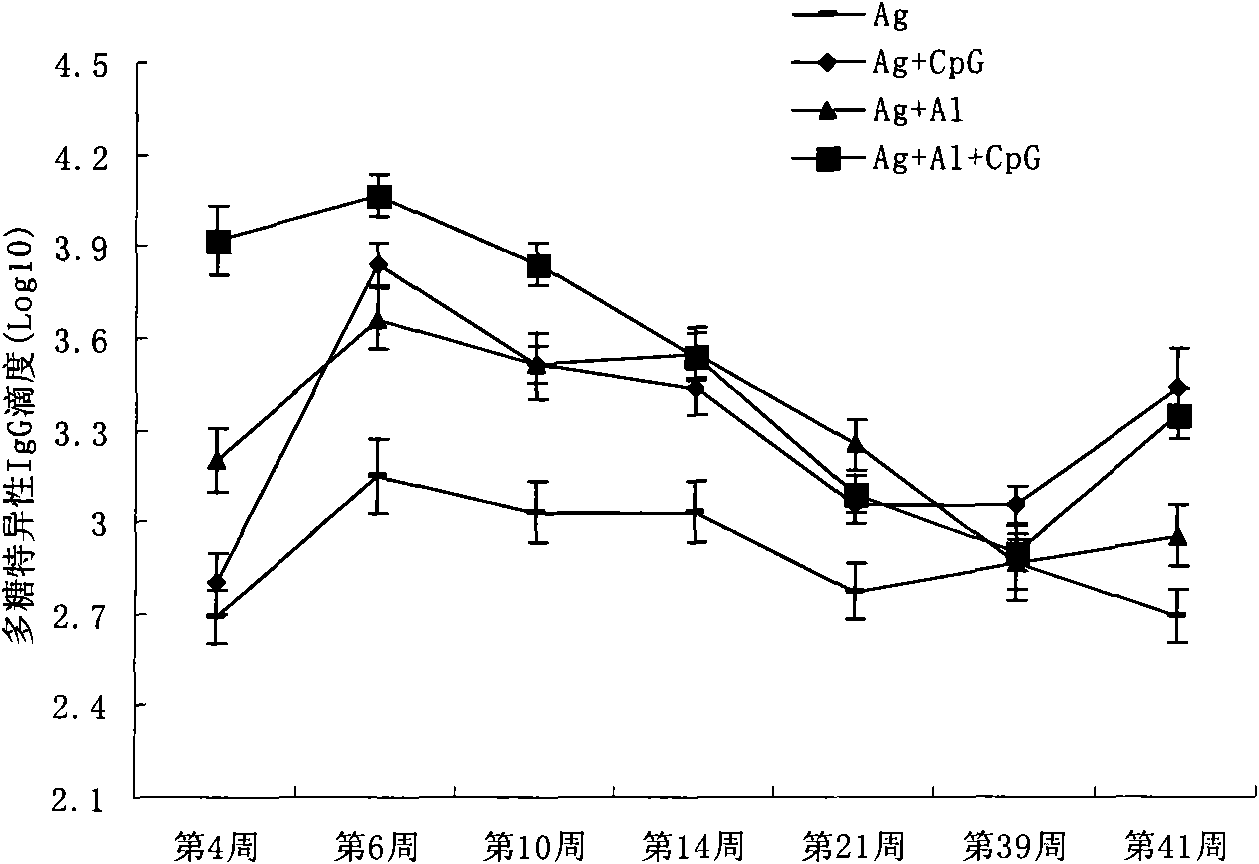
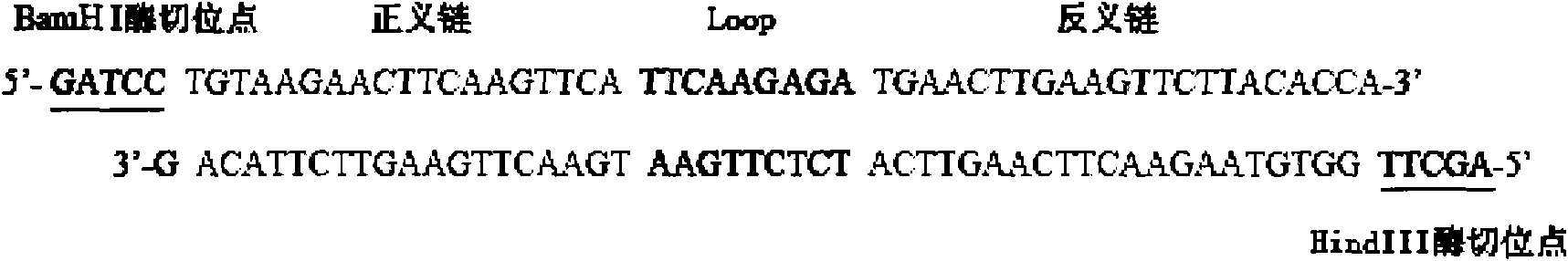
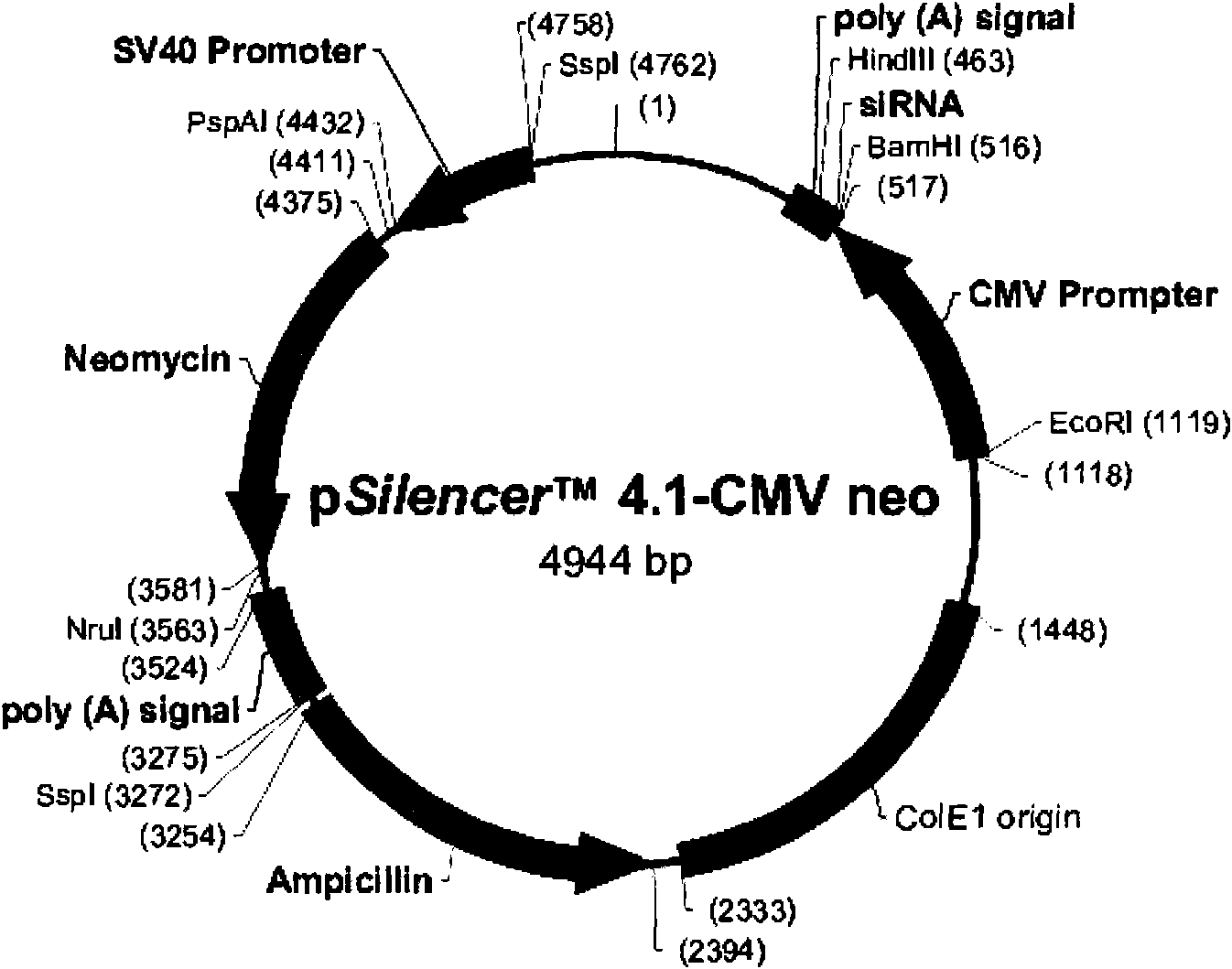
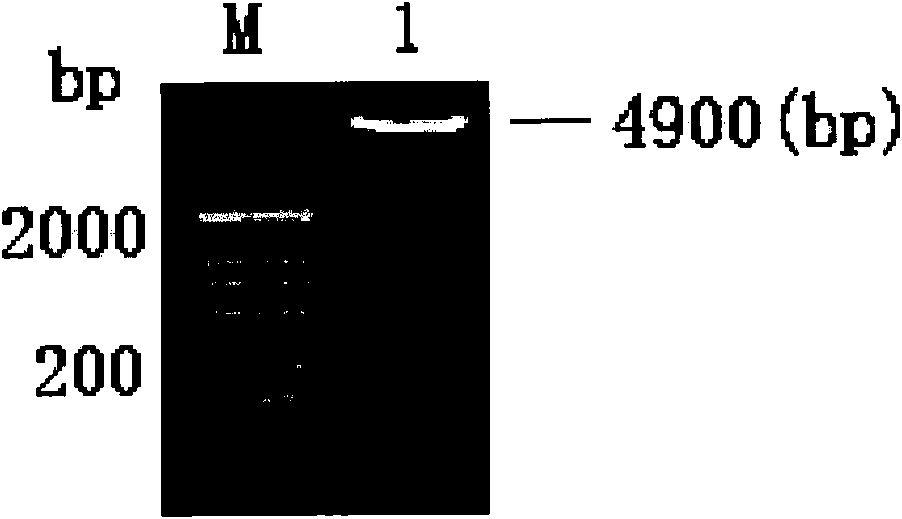
InactiveCN101818144AVector-based foreign material introductionDNA/RNA fragmentationRestriction Enzyme Cut SiteWestern blot
The invention belongs to the field of molecular biology, and discloses an siRNA segment capable of inhibiting mouse TLR9 expression and a vector built on the basis of the segment. A sequence of the siRNA segment is 5'-UGUAAGAACUUCAAGUUCA-3'; two Oligo single strands of which two corresponding ends have BamHI and Hind III restriction enzyme cutting sites are synthesized according to the sequence, and are annealed continuously and connected to the vector to build an interference vector pSilencerTM4.1-siRNA9.1. Specific examples, such as RT-PCR, western blot and the like, validate that the interference vector can be used for inhibiting the expression of a mouse Raw264.7 cell TLR9 and block a signal channel caused by the TLR9 successfully and specifically. Therefore, the siRNA9.1 sequence and the interference vector obtained by the siRNA9.1 sequence can be used for obtaining a large number of mouse cells with low TLR9 expression cells and blocking the TLR9 signal channel economically, fast and conveniently.
Owner:NANJING UNIV
Poliomyelitis vaccine
InactiveCN101559224AImprove immunityLow costViral antigen ingredientsAntiviralsSpecific immunityRibonucleotide synthesis
The invention relates to a poliomyelitis vaccine comprising an inactivated poliomyelitis virus used as an antigen, and a first adjuvant, wherein the first adjuvant is oligodeoxynucleotide comprising at least one non-methylation CpG dinucleotide, and the length of the oligodeoxynucleotide is 15-35 ribonucleotides. The adjuvant used for increasing the immunization effect of the inactivated poliomyelitis vaccine is a novel human vaccine adjuvant with favorable potential application prospect, and the chemical essence of the adjuvant is the oligodeoxynucleotide which is synthesized manually and contains the CpG dinucleotide. By a receptor TLR9 activated immune system on immune cells, the adjuvant reinforces specific immune response aiming at vaccine antigens. The immunization effect of a vaccine can be increased by adding the vaccine adjuvant into the vaccine, so that the dosage of the vaccine antigen can be reduced and the vaccine cost can be reduced finally.
Owner:NAT VACCINE & SERUM INST
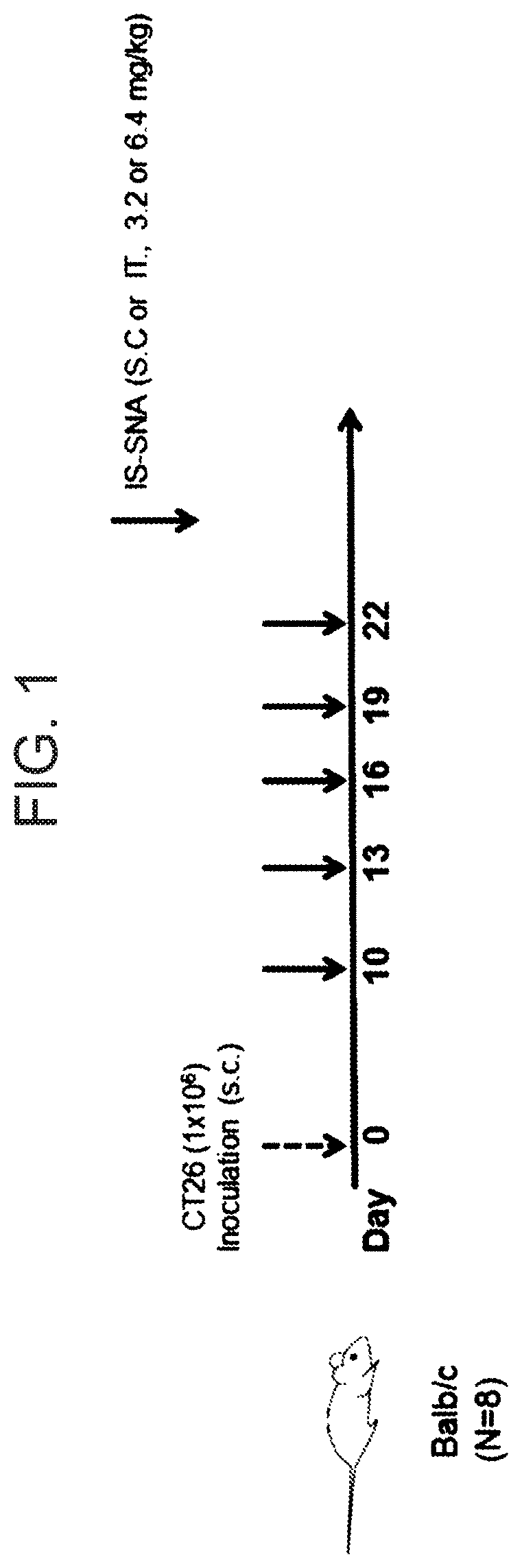
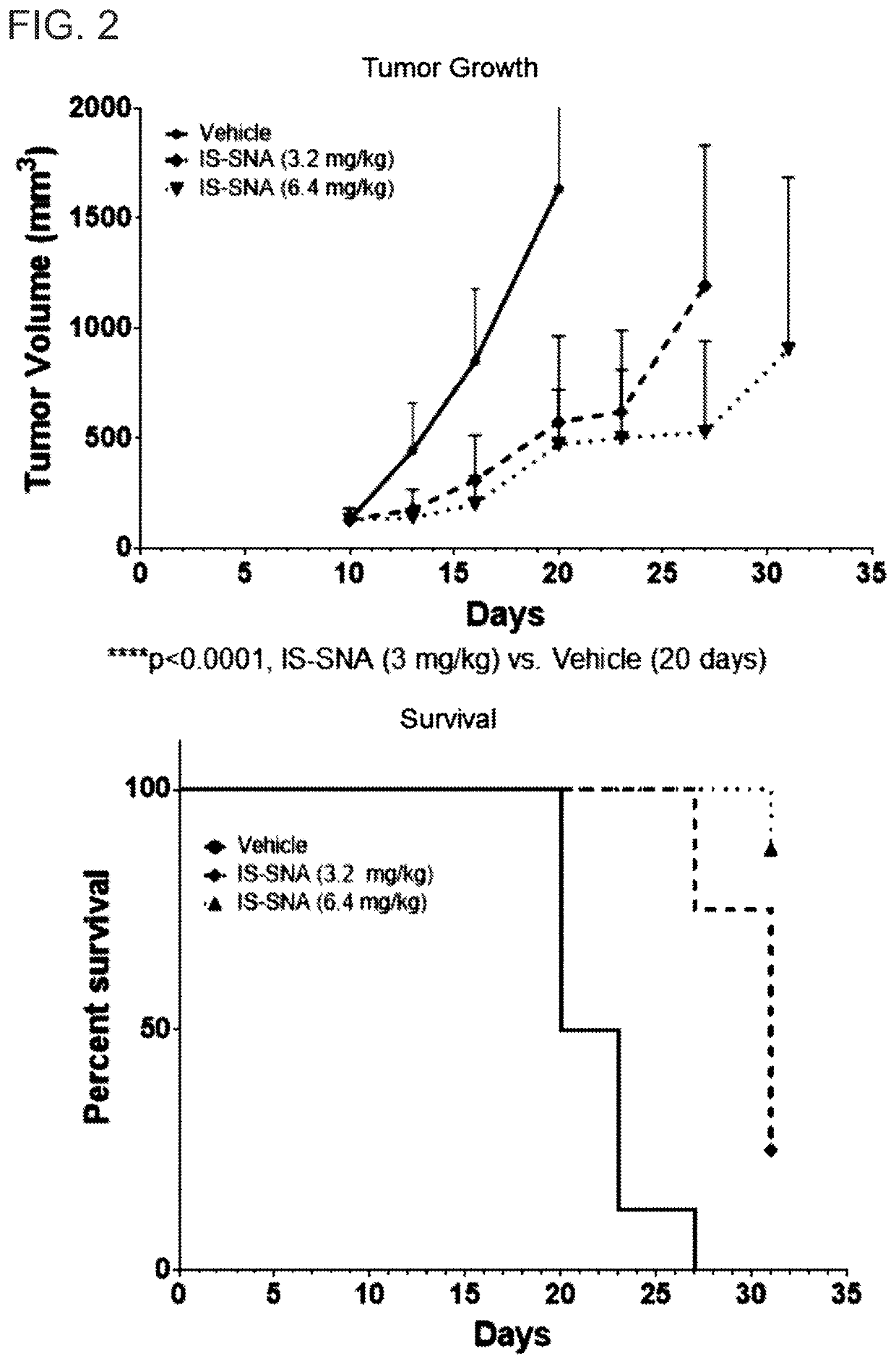
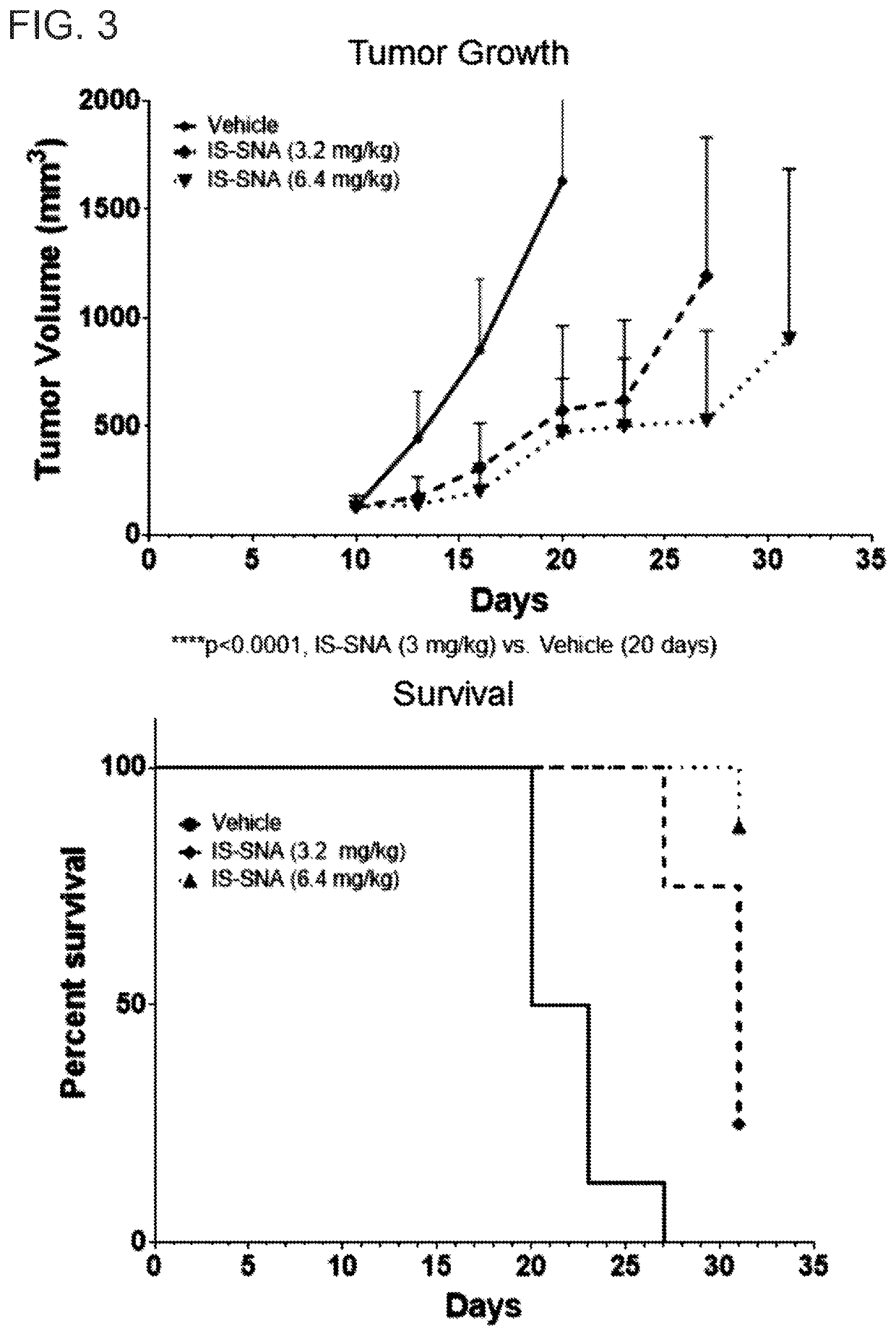
Tlr9-targeted spherical nucleic acids having potent antitumor activity
PendingUS20200248183A1Raise the ratioOrganic active ingredientsPharmaceutical delivery mechanismDiseaseSpherical nucleic acid
Aspects of the invention relate to immunostimulatory spherical nucleic acids (IS-SNA) for the treatment of a disorder, such as cancer. The IS-SNA may be administered together with a checkpoint inhibitor.
Owner:EXICURE INC
Treatment of liver cancer
InactiveUS20130315940A1Reduced activityReduce the amount requiredCompound screeningBiocideOncologyTLR7
The present invention derives from the finding that increased levels of Toll related receptor 9 (TLR9) and Toll related receptor 7 (TLR7) are associated with liver cancer and in particular hepatocellular carcinoma. Accordingly, the invention provides TLR9 and TLR7 antagonists for use in a method of treating liver cancer. The invention provides other strategies such as modulation of endosomal signalling by using compounds such as chloroquine to prevent or treat such liver cancer. The invention also provides agents capable of inducing an immune response against TLR9 and TLR7 for use in a method of treating liver cancer. The presence of TLR9 and TLR7 expression may be indicative of the presence of liver cancer and the amount of TLR9 and TLR7 expression may be indicative of the rate of growth or spreading of the cancer.
Owner:JALAN RAJIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
![Pyrrolo[3,2-c]pyridine derivatives as TLR inhibitors Pyrrolo[3,2-c]pyridine derivatives as TLR inhibitors](https://images-eureka.patsnap.com/patent_img/ad6747b1-7056-4231-b2a4-e2bbf5d791d5/US09643967-20170509-D00001.png)
![Pyrrolo[3,2-c]pyridine derivatives as TLR inhibitors Pyrrolo[3,2-c]pyridine derivatives as TLR inhibitors](https://images-eureka.patsnap.com/patent_img/ad6747b1-7056-4231-b2a4-e2bbf5d791d5/US09643967-20170509-D00002.png)
![Pyrrolo[3,2-c]pyridine derivatives as TLR inhibitors Pyrrolo[3,2-c]pyridine derivatives as TLR inhibitors](https://images-eureka.patsnap.com/patent_img/ad6747b1-7056-4231-b2a4-e2bbf5d791d5/US09643967-20170509-D00003.png)
![Pyrrolo[3,2-c]pyridine derivatives as tlr inhibitors Pyrrolo[3,2-c]pyridine derivatives as tlr inhibitors](https://images-eureka.patsnap.com/patent_img/e6c86b47-fe37-4b15-b1aa-df880fd05eca/US20170008885A1-20170112-D00001.png)
![Pyrrolo[3,2-c]pyridine derivatives as tlr inhibitors Pyrrolo[3,2-c]pyridine derivatives as tlr inhibitors](https://images-eureka.patsnap.com/patent_img/e6c86b47-fe37-4b15-b1aa-df880fd05eca/US20170008885A1-20170112-D00002.png)
![Pyrrolo[3,2-c]pyridine derivatives as tlr inhibitors Pyrrolo[3,2-c]pyridine derivatives as tlr inhibitors](https://images-eureka.patsnap.com/patent_img/e6c86b47-fe37-4b15-b1aa-df880fd05eca/US20170008885A1-20170112-D00003.png)