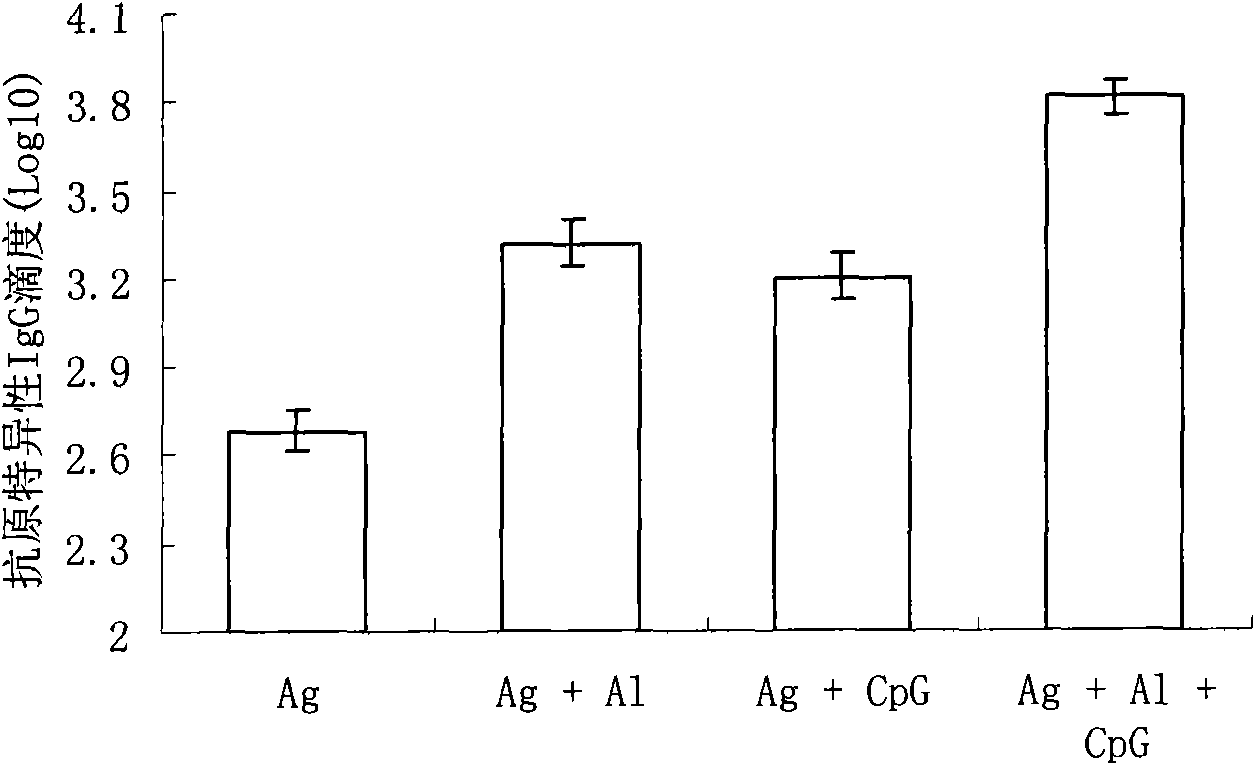Poliomyelitis vaccine
A polio and vaccine technology, applied in biochemical equipment and methods, viral antigen components, microorganisms, etc., can solve problems such as the joint action of polioviruses that are not involved, and achieve the enhancement of specific immune responses, reduction of vaccine costs, The effect of reducing the dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1. Preparation of Inactivated Poliovirus
[0036] Cells: African green monkey kidney passage cell line (Vero cell line) was used. The cells were isolated from the kidney of African green monkeys by Japanese scholars in 1963. The 112th generation was sent to the American Type Culture Collection (ATCC). After identification, the cells had no tumorigenicity, no bacteria, mold and mycoplasma contamination, no virus contamination, and were suitable for the production of biological products.
[0037] In December 1988, the Beijing Institute of Biological Products obtained a second bottle of 117-generation Vero cells (numbered CCL81) from ATCC in the United States, and immediately frozen them in liquid nitrogen as the original seeds. The main cell bank was prepared by subculture and expansion of an original seed, and the working cell bank was prepared by subculture and expansion of a master seed bank cell. The verification of the main cell bank and the working cell ba...
Embodiment 2
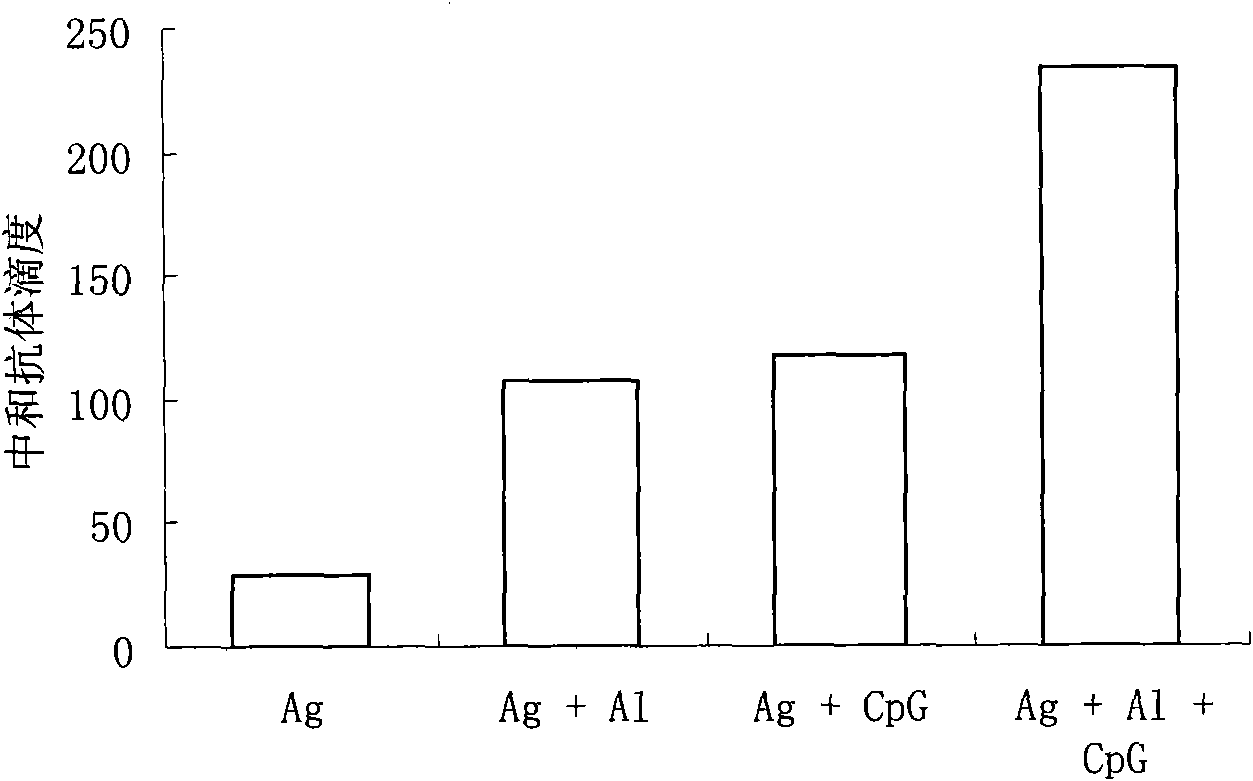
[0047] Example 2 CpG-ODN can enhance the immune response of mice to Sabin II type inactivated polio vaccine
[0048] The inactivated poliovirus used in this example was prepared according to the method described in Example 1, and is hereinafter referred to as Ag for short.
[0049] The CpG-ODN used in this example was designed by the inventors, and its sequence is as follows: 5′-TCGTTCGTTCGTTCGTTCGTT-3′, which was artificially synthesized by Shanghai Sangon Biotechnology Co., Ltd., and subjected to full-chain thio modification, and polyacrylamide gel electrophoresis Purified, dissolved in normal saline, and stored in a -20°C refrigerator for later use.
[0050] The mice used in this example are Balb / c mice, female, 6-8 weeks old, purchased from the Experimental Animal Center of the Chinese Academy of Medical Sciences.
[0051] Divide the Balb / c mice into four groups, namely "Ag group", "Ag+Al group", "Ag+CpG group" and "Ag+Al+CpG group", with 8 mice in each group, each The m...
Embodiment 3
[0060] Example 3. CpG-ODN can reduce the antigen dosage of Sabin II type inactivated polio vaccine
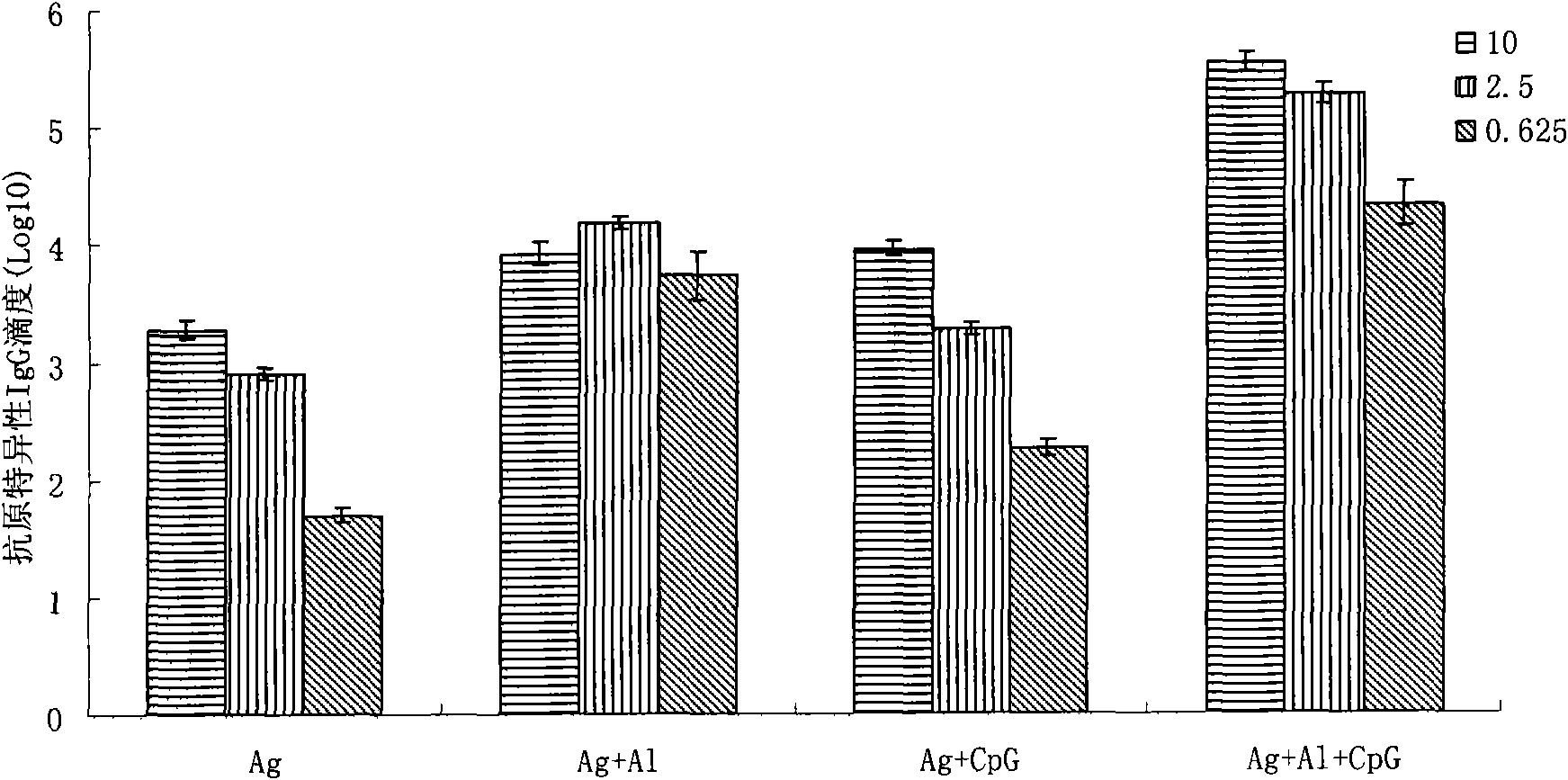
[0061] Balb / c mice were immunized according to the same method as in Example 2, and then specific IgG antibody titers and neutralizing antibody titers were detected to determine the antigen dosage of CpG-ODN to Sabin II type inactivated polio vaccine The difference is that the amount of antigen used in inactivated polio vaccine is the original dose (10 μg Ag / mouse), one-quarter dose (2.5 μg Ag / mouse) and one-sixteenth Dose (0.625 μg of Ag / mouse). See the results separately Figure 2a and 2b .
[0062] Figure 2a The results shown are the Sabin II poliovirus-specific IgG antibody titers at the 4th week after immunization. Depend on Figure 2a It can be seen that the "Ag+CpG group" only needs a quarter of the dose of Ag to achieve the immune effect of the original antigen dose of the "Ag group". The "Ag+Al+CpG group" had the strongest immune response, and only one sixteent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com