Patents
Literature
61 results about "Platelet concentrate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Platelet concentrate A blood product prepared from a single donor, which transiently increases platelet count by 5-10 x 109/L/m2 body surface area (5–10,000/µL) if thrombocytopaenia (decreased platelet count) is not due to increased platelet destruction.
Platelet suspensions and methods for resuspending platelets
Platelet suspensions and methods for resuspending platelet concentrates are disclosed. The platelet concentrates are resuspended by combining a platelet concentrate with a hypertonic solution of either sodium chloride or potassium chloride. The resuspended platelets may be stored and / or administered to a patient.
Owner:FENWAL
Method for treatment and storage of platelets
ActiveUS20100009334A1Reducing or eliminating the atmosphere comprising xenonBiocideDead animal preservationXenonRefrigerated temperature
Provides are improved methods for storing platelets and compositions that contain stored platelets for use in transfusions. The method entails obtaining a platelet concentrate from blood obtained from an individual and holding the platelet concentrate in at refrigerated temperatures under an atmosphere having a pressure of from 3.5 to 5 bars comprising more than 65% xenon and for at least one week. Also provided is a refrigerated composition that contains a platelet concentrate, wherein the platelet concentrate contains xenon, and wherein the platelet concentrate has been isolated from an individual for at least seven days.
Owner:RICH TECH HLDG CO LLC
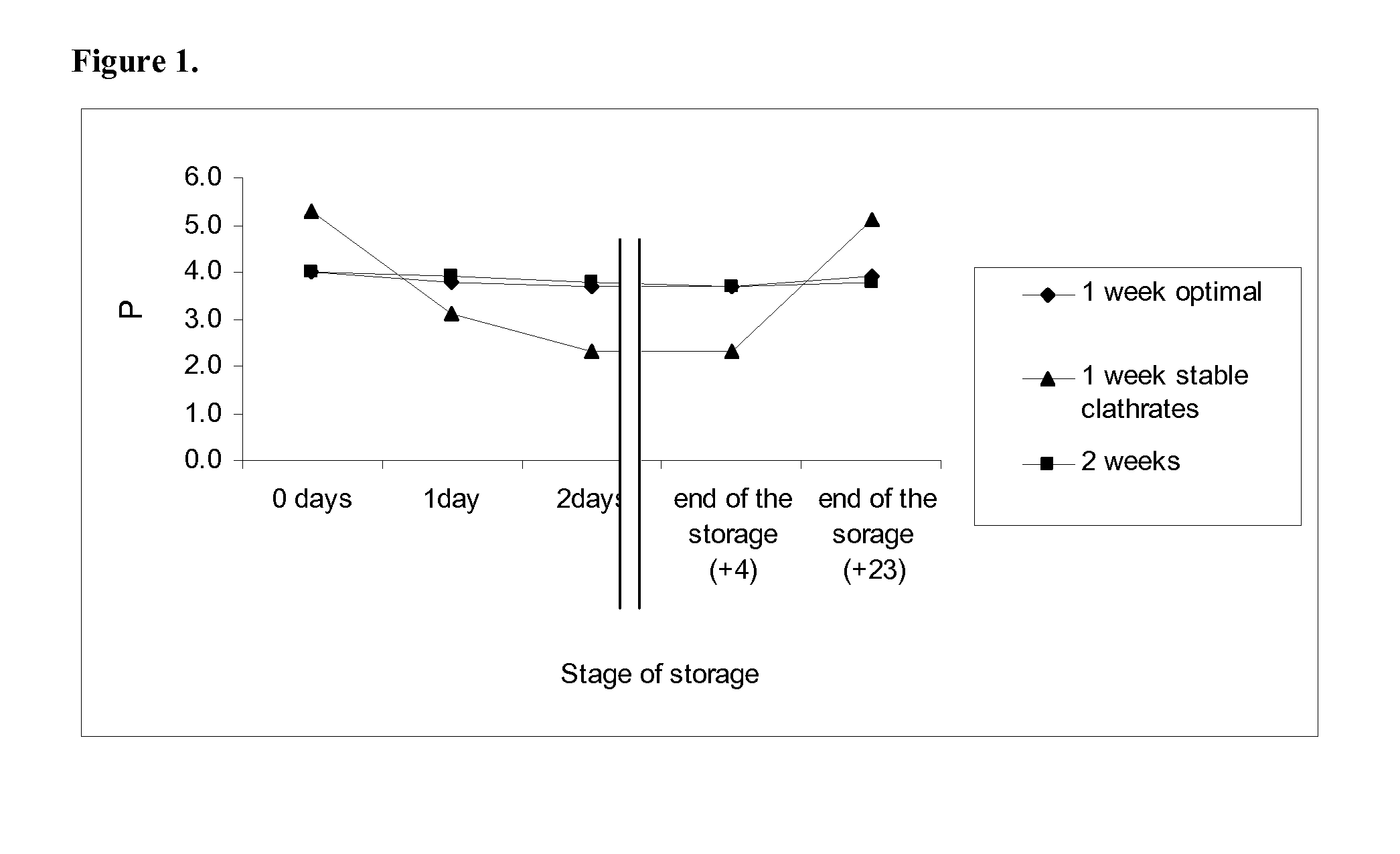
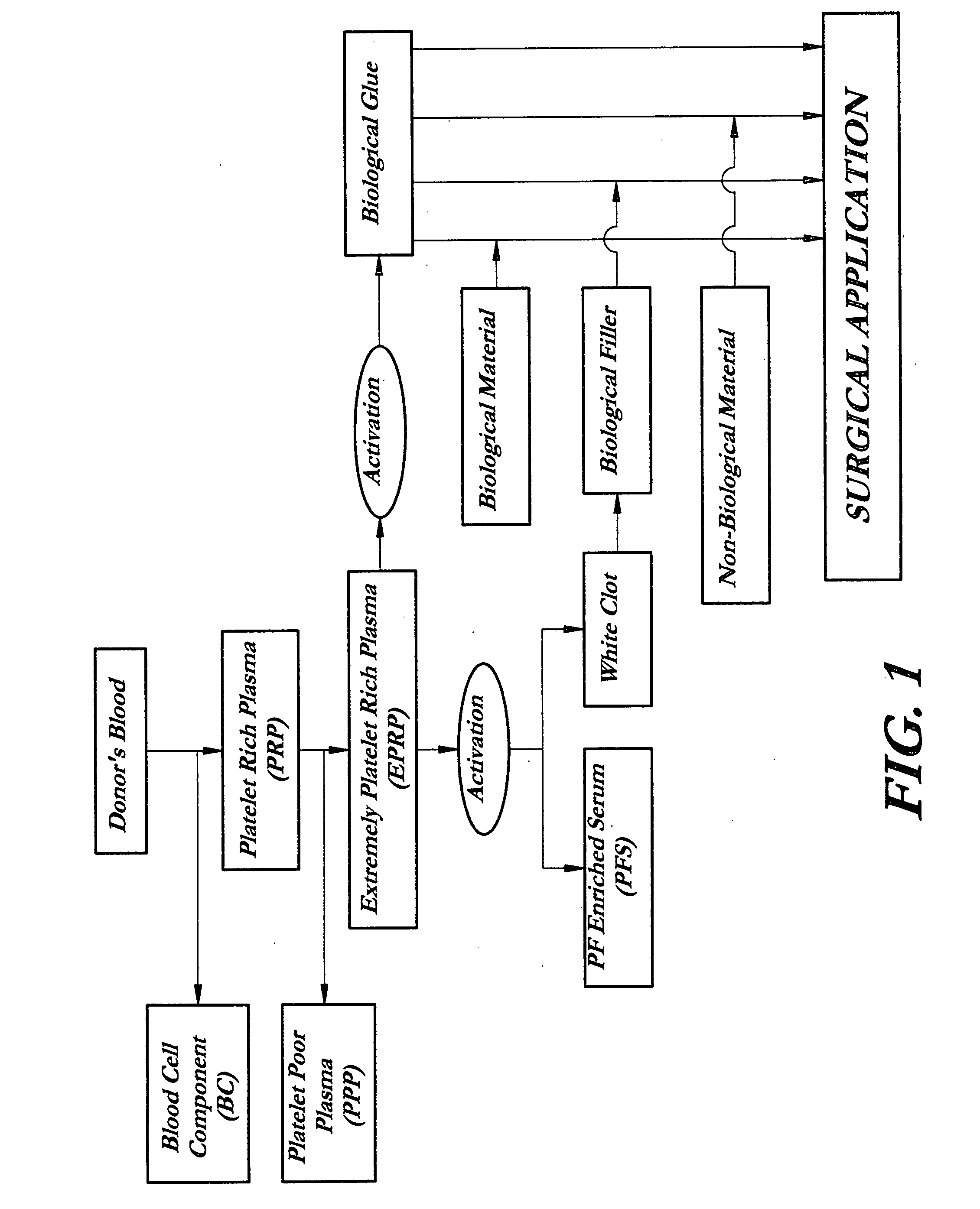
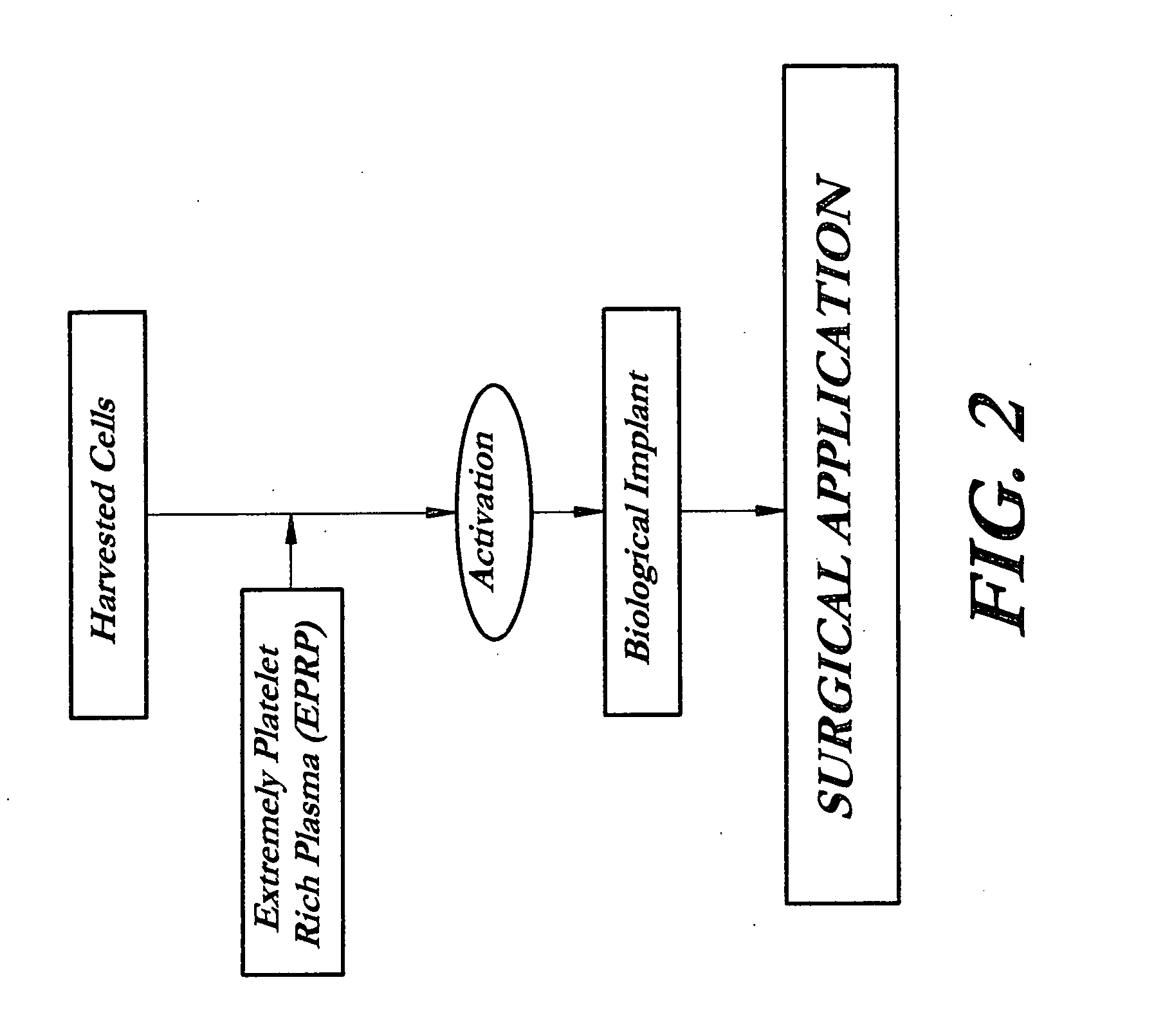
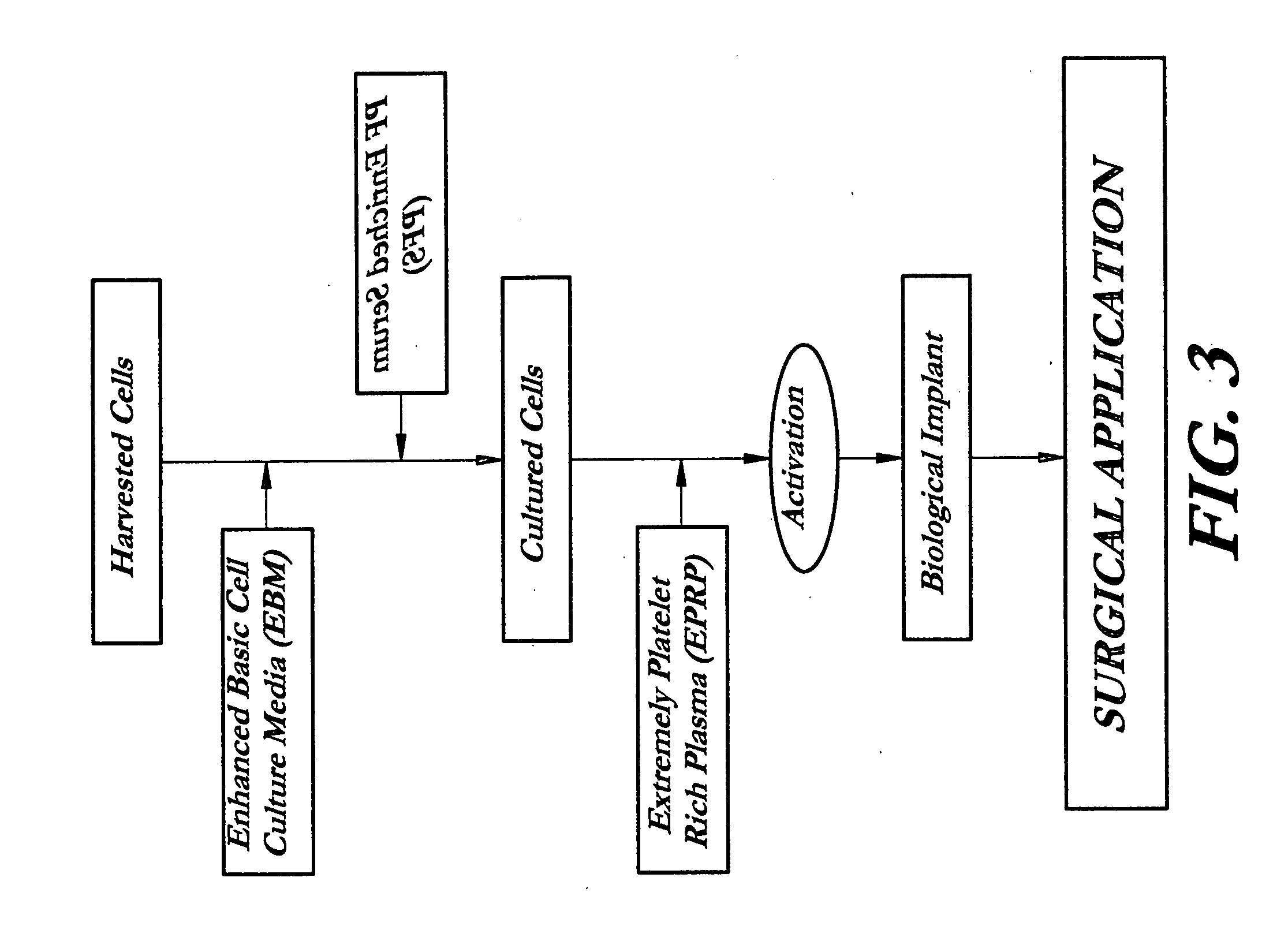
Composition and procedure for tissue creation, regeneration and repair by a cell-bearing biological implant enriched with platelet concentrate and supplements
InactiveUS20070141036A1Fill the voidIncrease the number of cellsBiocideCulture processThrombusIn vivo
A composition and method for enhancing tissue growth, regeneration, and repair includes a Biological Glue formed by extraction of an Extremely Platelet Rich Plasma (EPRP) derived from whole blood, and subsequent activation and clotting. The Biological Glue may be utilized alone to fill defects or may be used as an adhesive agent for other biological and non-biological materials. These materials may include processed thrombus derived from the activation of EPRP. Additionally, the Extremely Platelet Rich Plasma may be impregnated with directly harvested or cultured cells, including stem cells, or other materials, prior to activation, to form a Biological Implant that may be implanted in vivo. A Platelet Factor Enriched Serum (PFS) derived from the activation of the Extremely Platelet Rich Plasma (EPRP) may be added to the cell cultures in preparation of a Biological Implant, in order to provide additional growth factors that speed the development of the cell cultures.
Owner:GORROCHATEGUI BARRUETA ALBERTO
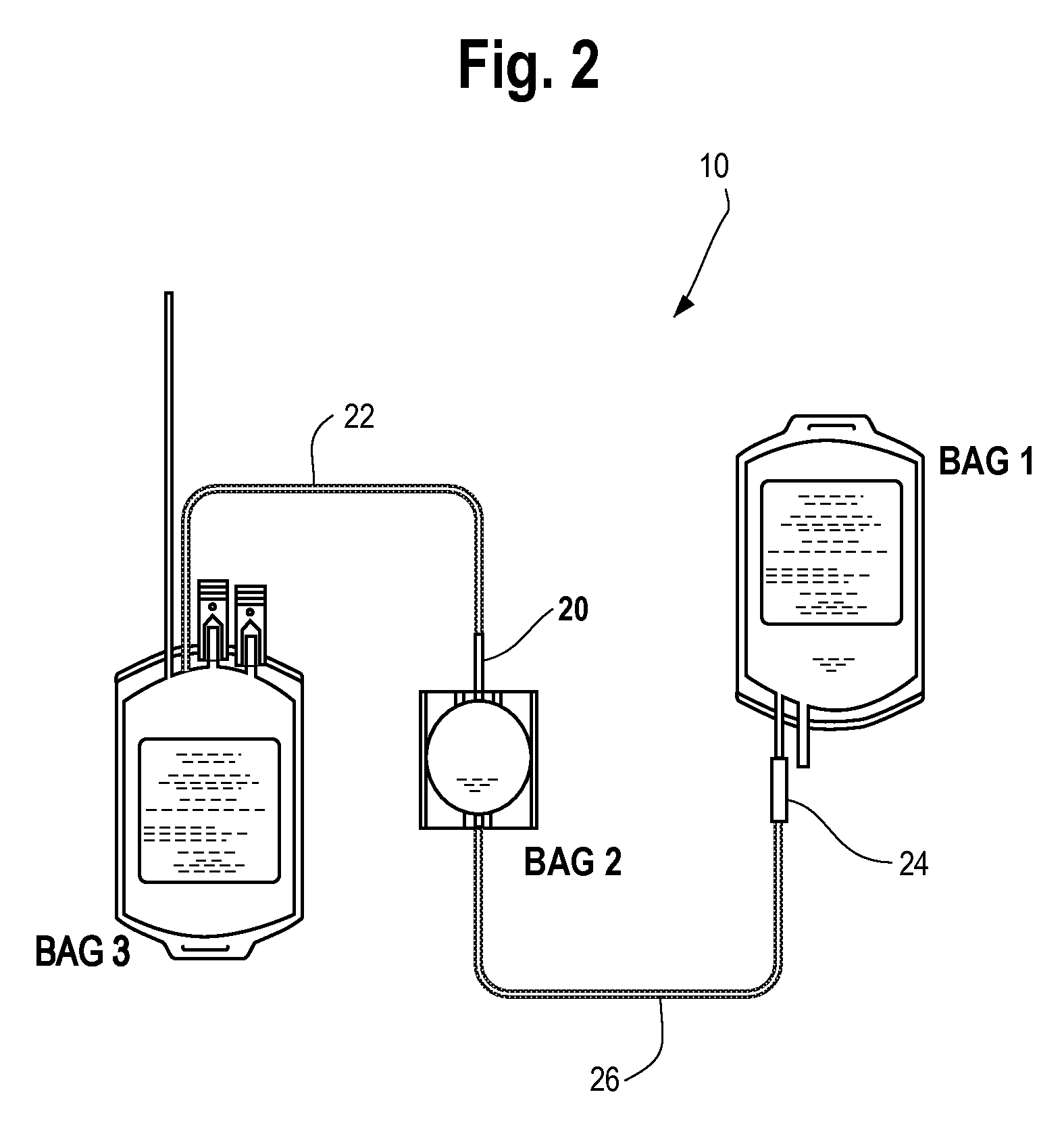
Blood processing systems and methods
InactiveUS6899666B2Easy to handleLiquid separation auxillary apparatusOther blood circulation devicesRotational axisMedicine
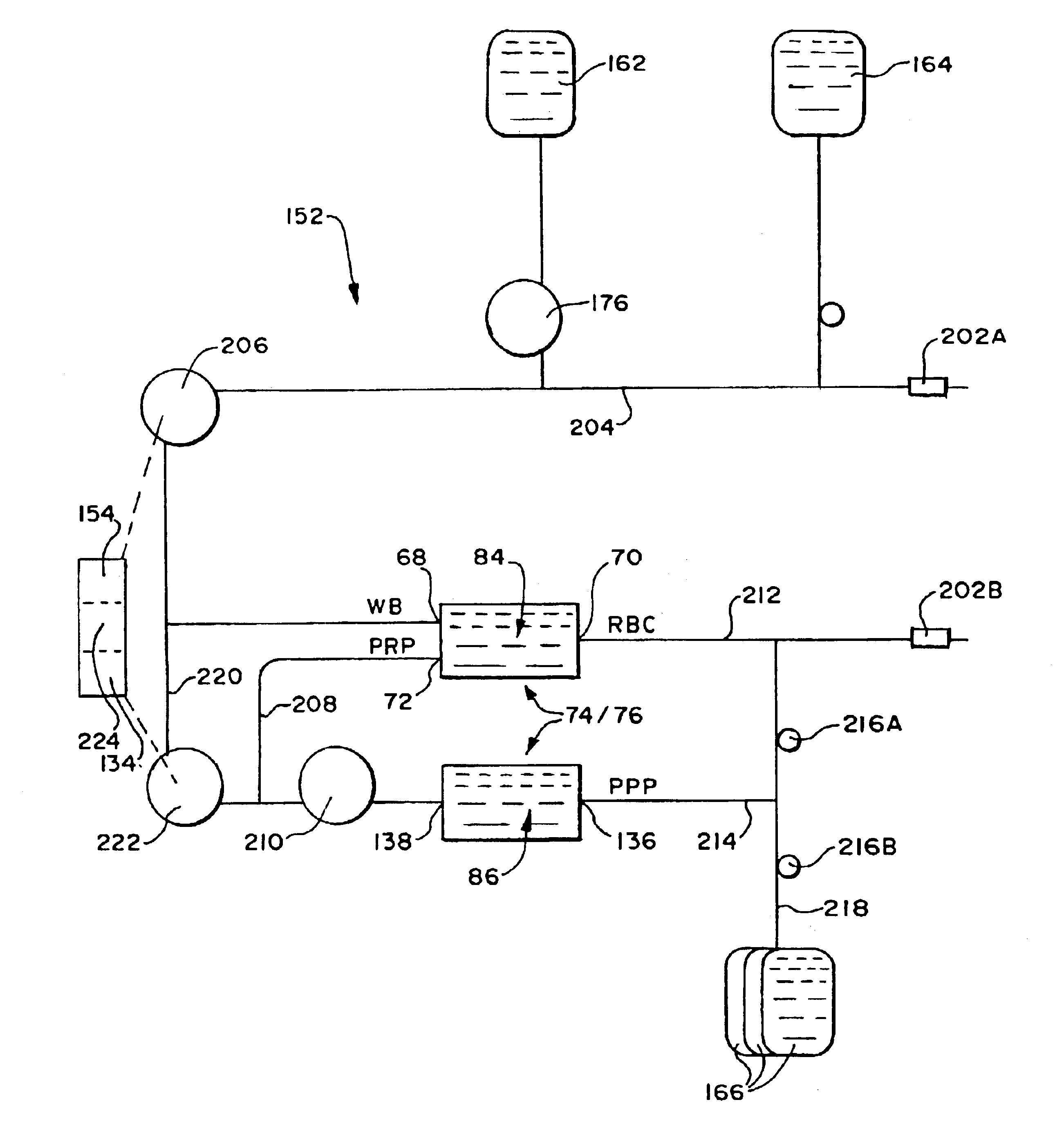
Systems and methods centrifugally separate whole blood into red blood cells, plasma, and a platelet concentrate. The systems and methods rotate a first rotating separation zone about a rotational axis, to separate whole blood into red blood cells and plasma constituent carrying platelets. Red blood cells separated are directed in a first circumferential flow direction toward a terminal wall, where blood flow is halted. Surface hematocrit is successively increased in the first circumferential flow direction by separating the plasma constituent from the red blood cells. Separated red blood cells are directed from the first rotating separation zone through a path where the surface hematocrit is the most. Plasma constituent separated is directed in a second circumferential flow direction opposite to the first circumferential flow direction toward a different region in the first rotating separation zone, where the surface hematocrit is the least. The systems and methods separate the plasma constituent into platelet concentrate and plasma in a second rotating separation zone.
Owner:FENWAL
Blood bag system and process for the inactivation of pathogens in platelet concentrates by use of the blood bag system
InactiveUS20100133203A1Reduction of therapeutic qualityAvoid accessDiagnosticsSurgeryWhite blood cellUltraviolet
The present invention relates to a blood bag system, a method for its manufacture, and a process for reducing pathogens and leucocytes in biological fluids in particular in therapeutic quantities of platelet concentrates (PC) contained in the blood bag system, using UV-light and agitation, wherein part of the plasma of the PC is optionally exchanged against a platelet additive solution.
Owner:MACO PHARMA SA
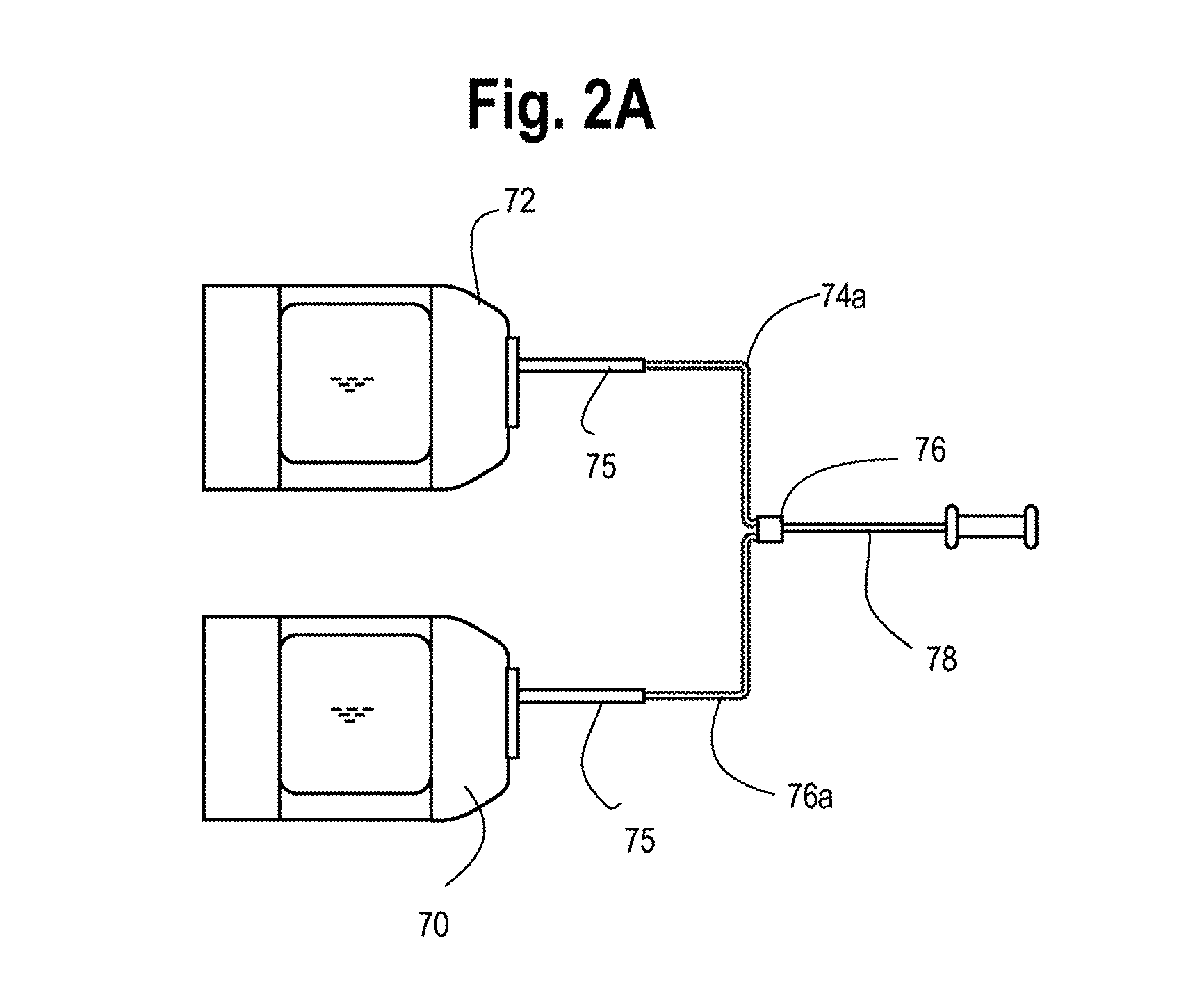
Platelet concentration syringe kit
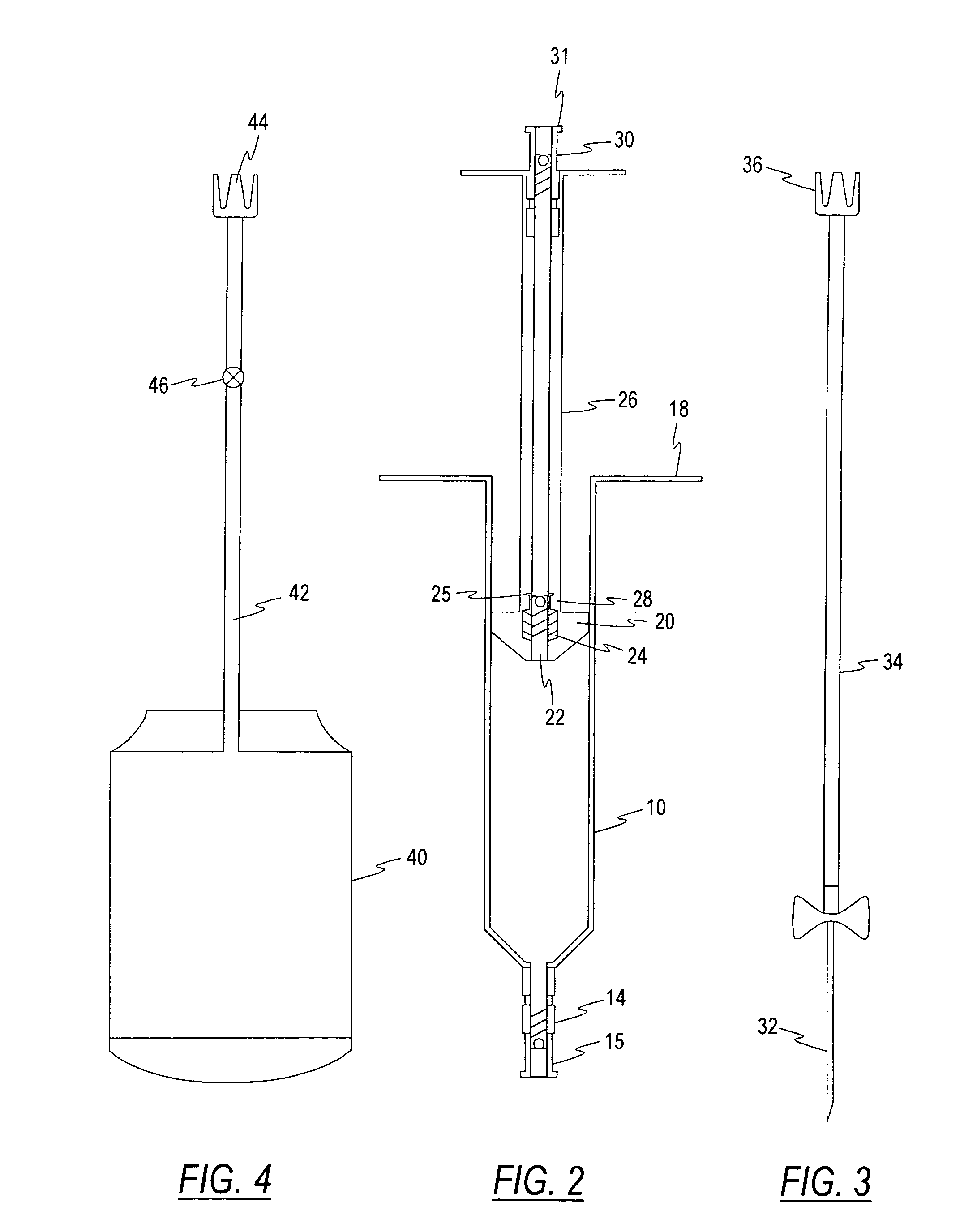
InactiveUS7452344B2Accurate separationPrevent coagulationOther blood circulation devicesAutomatic syringesCentrifugationBiomedical engineering
A single-use system for separating blood and producing platelet concentrates includes an elongated container for receiving blood from a patient. The container has a movable plunger mounted within the blood container for expressing blood fractions separated during centrifugation of the container through a first port mounted at one end of said container, a second port mounted on the plunger, and a third port mounted on a plunger rod attached to the plunger.
Owner:BIOMET 3I LLC
Blood component collection method
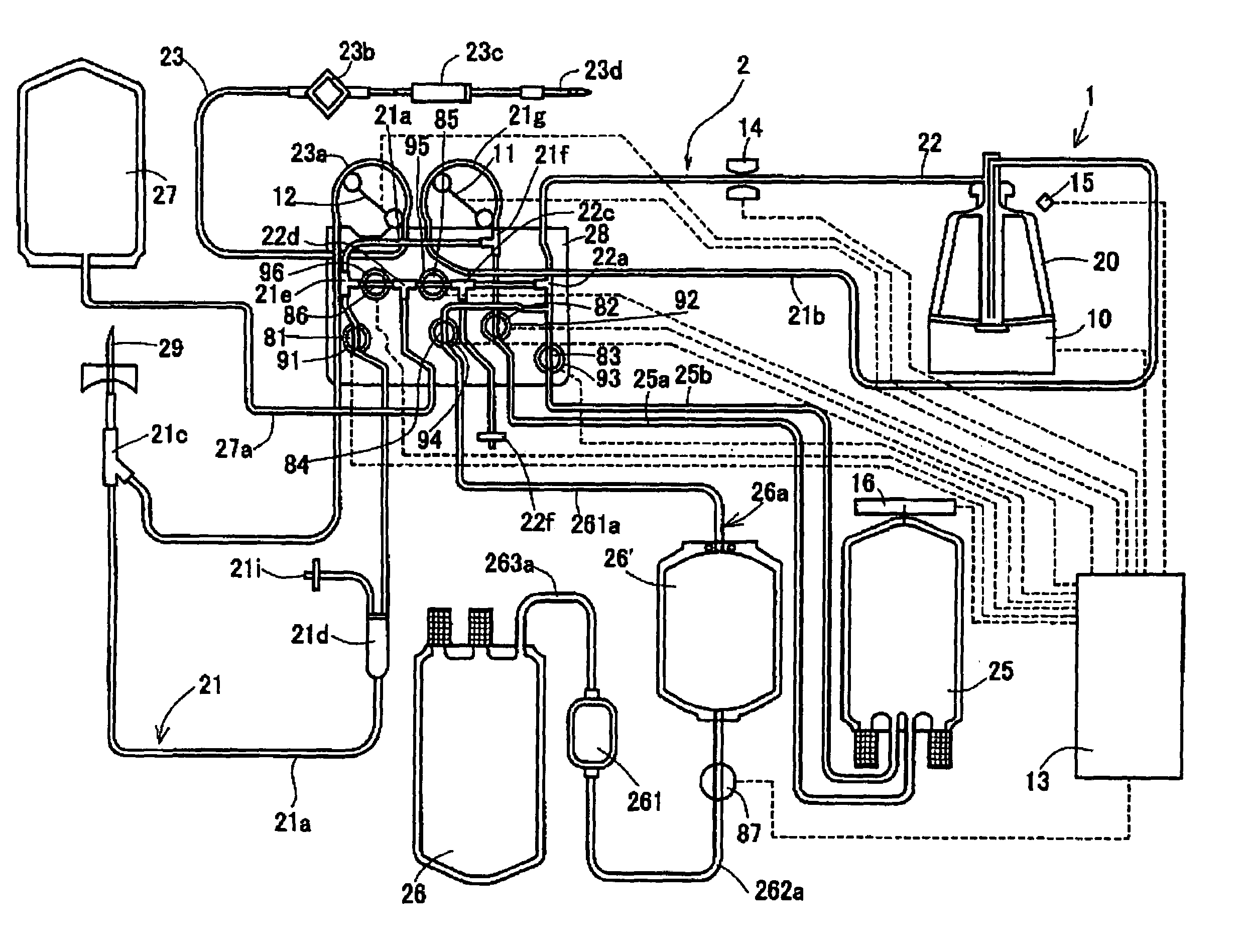
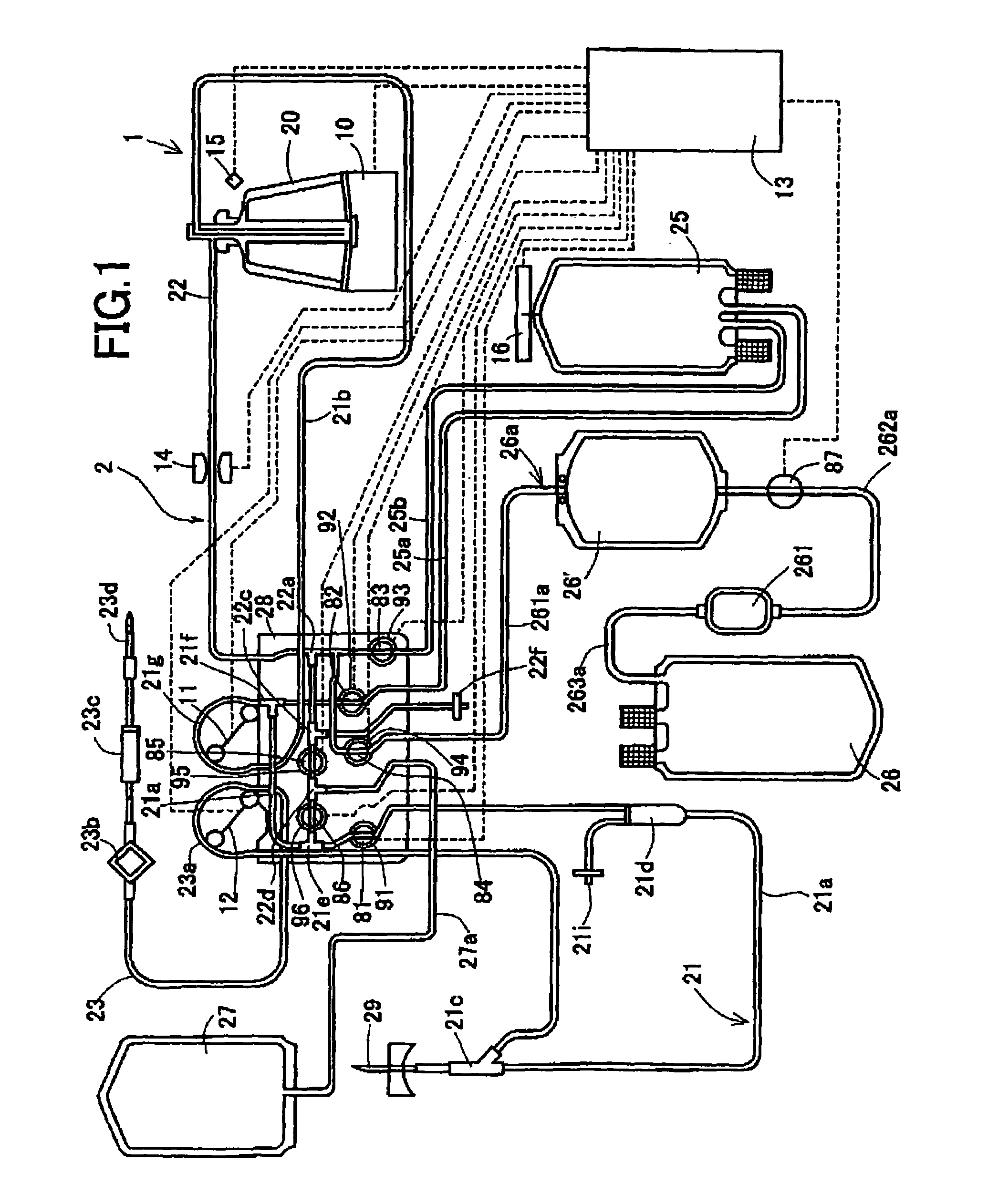
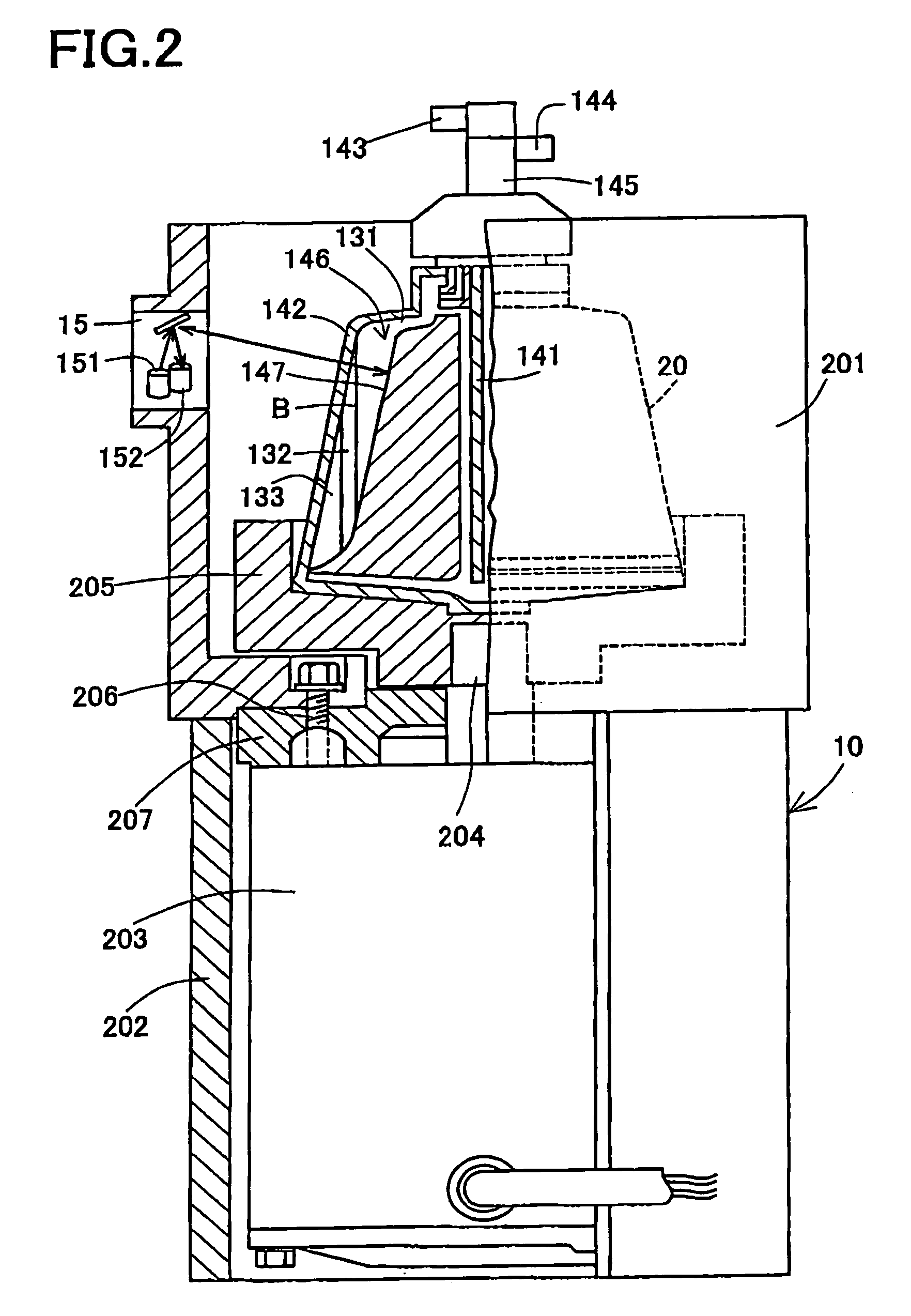
InactiveUS7186231B2Quality improvementOther blood circulation devicesHaemofiltrationBlood componentWhite blood cell
An object is to provide a blood component collection system capable of collecting a desired blood component of high quality. A blood component collection system 1 comprises a centrifugal separator 20 centrifuging blood into a plurality of components, first line 21 for feeding the blood to the centrifugal separator 20, a second line 22 for releasing the blood component from the centrifugal separator 20, a temporary reservoir bag 26′ for temporarily reserving the platelet concentrate, a leukoreduction filter 261 for separating and reducing leukocyte from the platelet concentrate, and a platelet collection bag 26 for reserving the platelet concentrate having passed through the leukoreduction filter 261. The blood component collection system 1 is constructed to feed the platelet concentrate in the temporary reservoir bag 26′ to the platelet collection bag 26 through the leukoreduction filter 261, and then, feed plasma and collect the platelet remaining in the leukoreduction filter 261 (platelet concentrate from which leukocyte have been reduced) together with the plasma into the platelet collection bag 26.
Owner:TERUMO KK
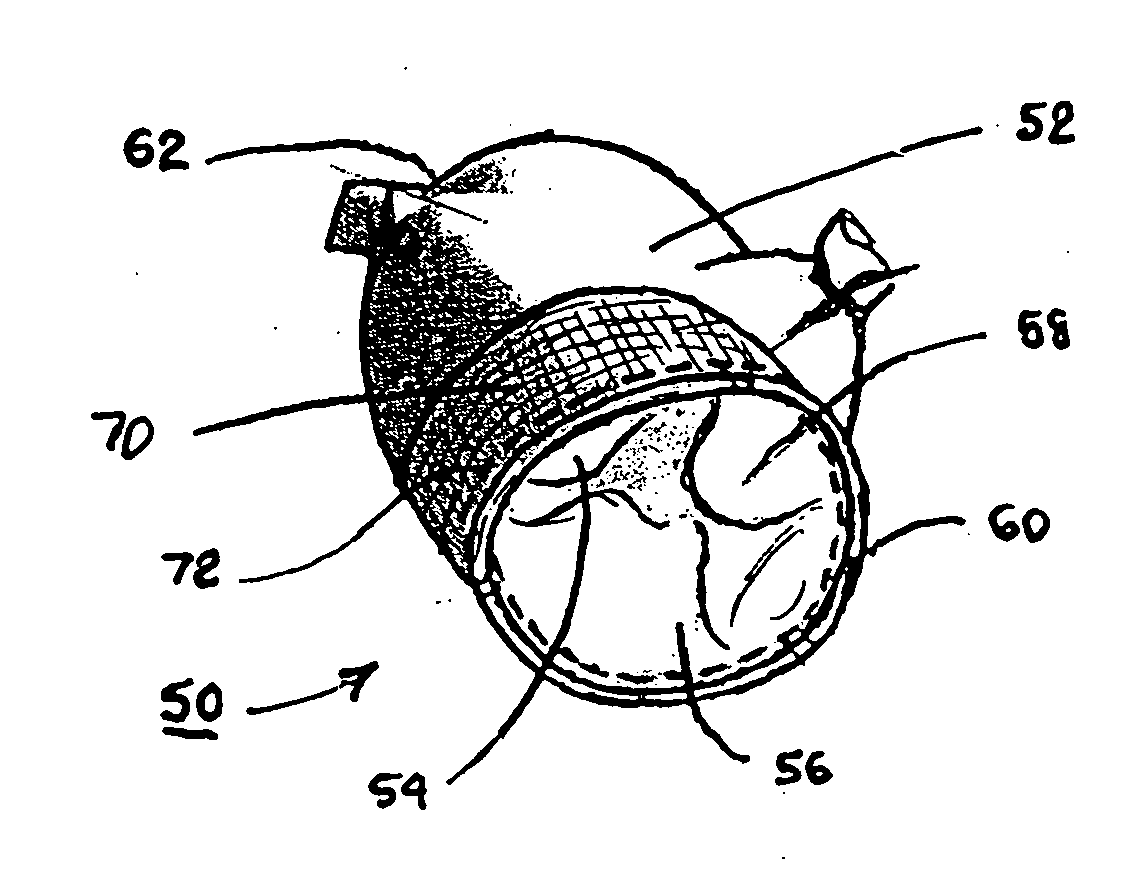
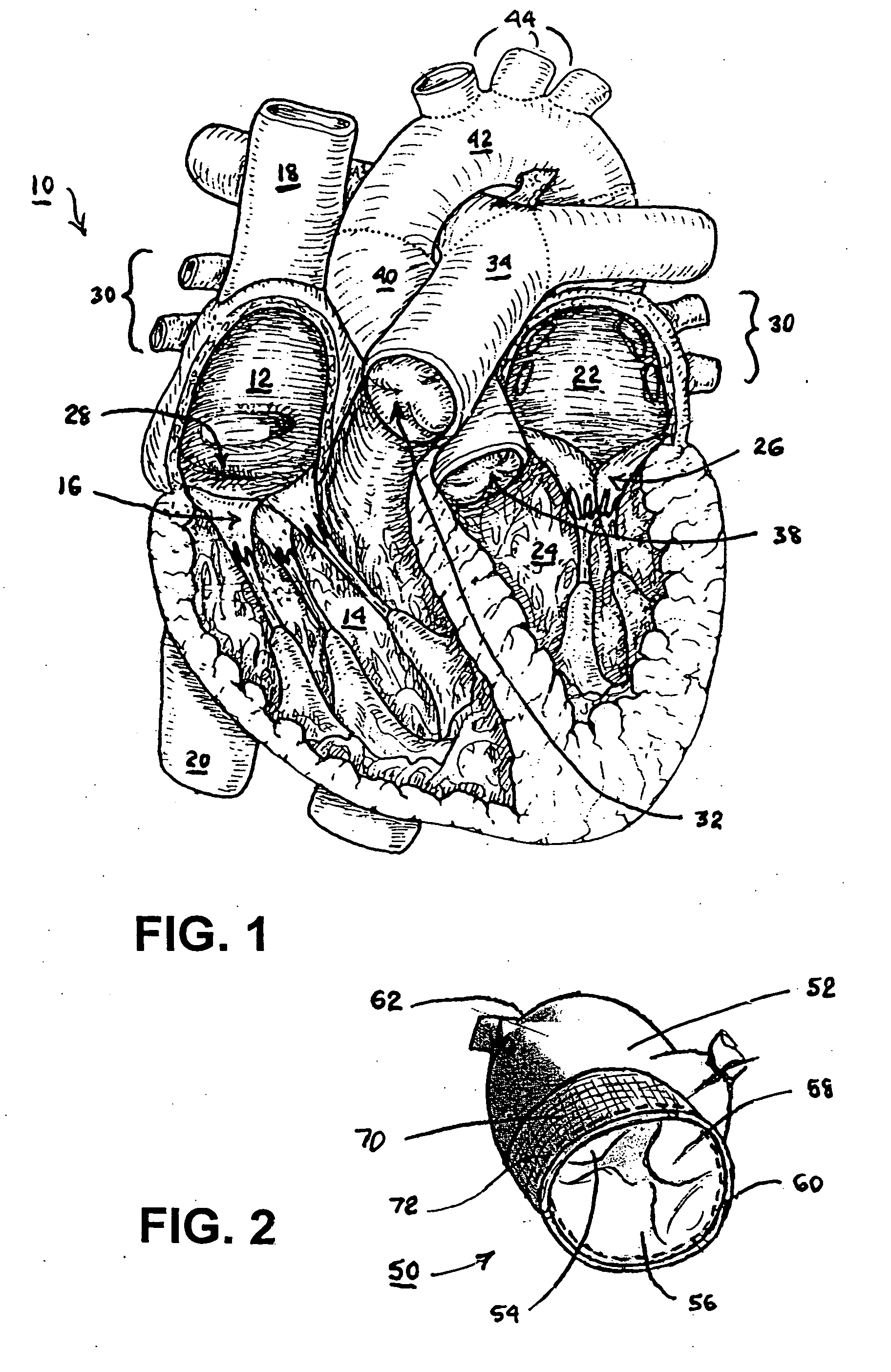
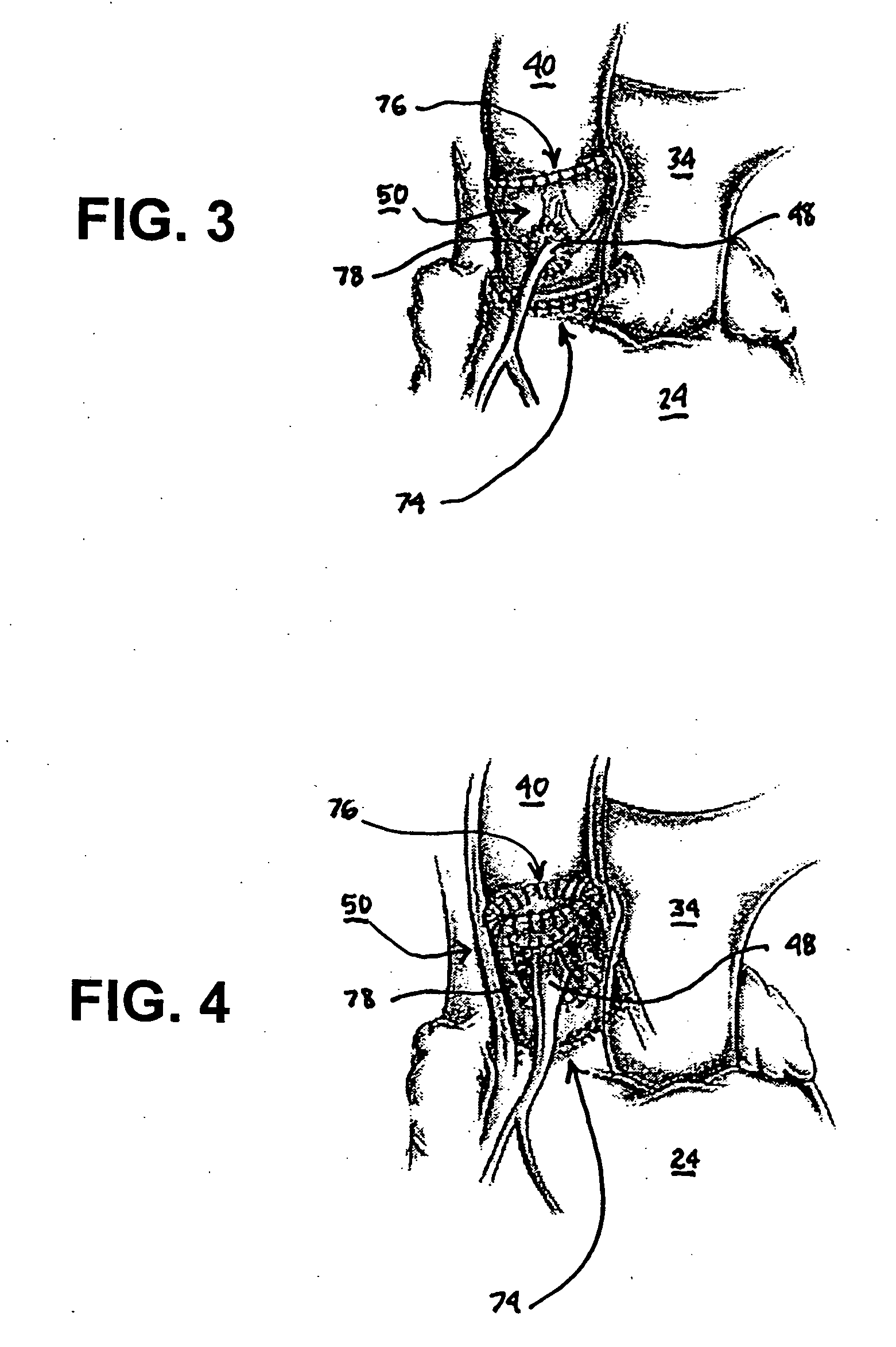
Fibrin sealants and platelet concentrates applied to effect hemostasis at the interface of an implantable medical device with body tissue
InactiveUS20060190017A1Stem bleedingAdvantageously employedHeart valvesAbsorbent padsParenchymaInternal bleeding
Surgical methods of and kits for applying and stabilizing a mass of fibrin sealant or platelet concentrate at the site of surgical attachment of an implantable medical device to effect hemostasis to stem internal bleeding at the site of surgical attachment are disclosed. A mass of fibrin sealant or platelet concentrate is applied onto a porous fabric, whereby the mass is supported in the interstices or pores of the fabric, and the supported mass is applied against the site of high pressure blood leakage. The supported mass achieves hemostasis as it does not wash away from the site. The present invention is particularly useful to effect hemostasis at sutures and suture holes extending through thin-walled tissue valves and grafts when such tissue valves or grafts are sutured in place, particularly at high blood pressure sites as at the valve annulus of the aortic valve or the aorta.
Owner:ARTERIOCYTE MEDICAL SYST
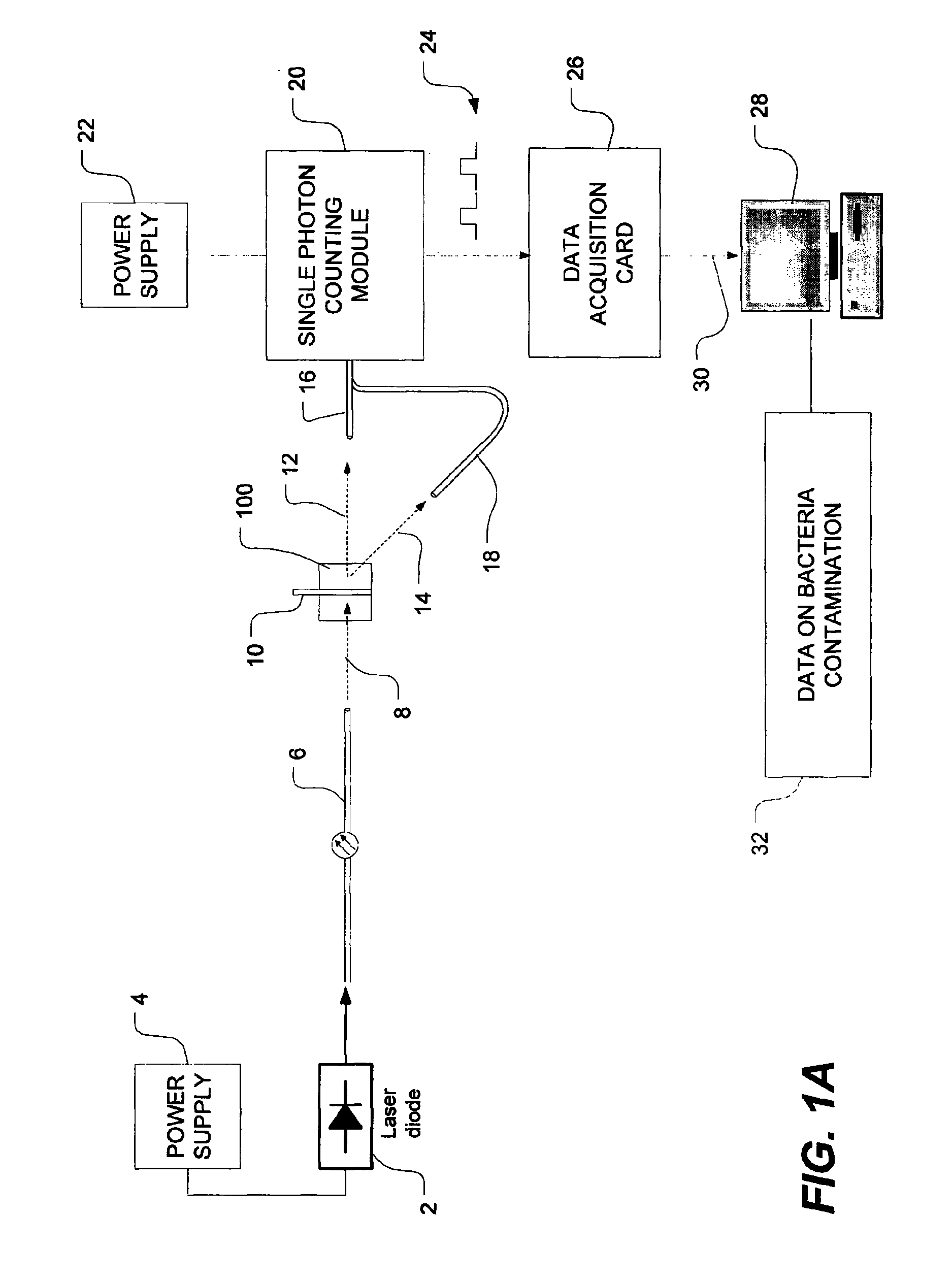
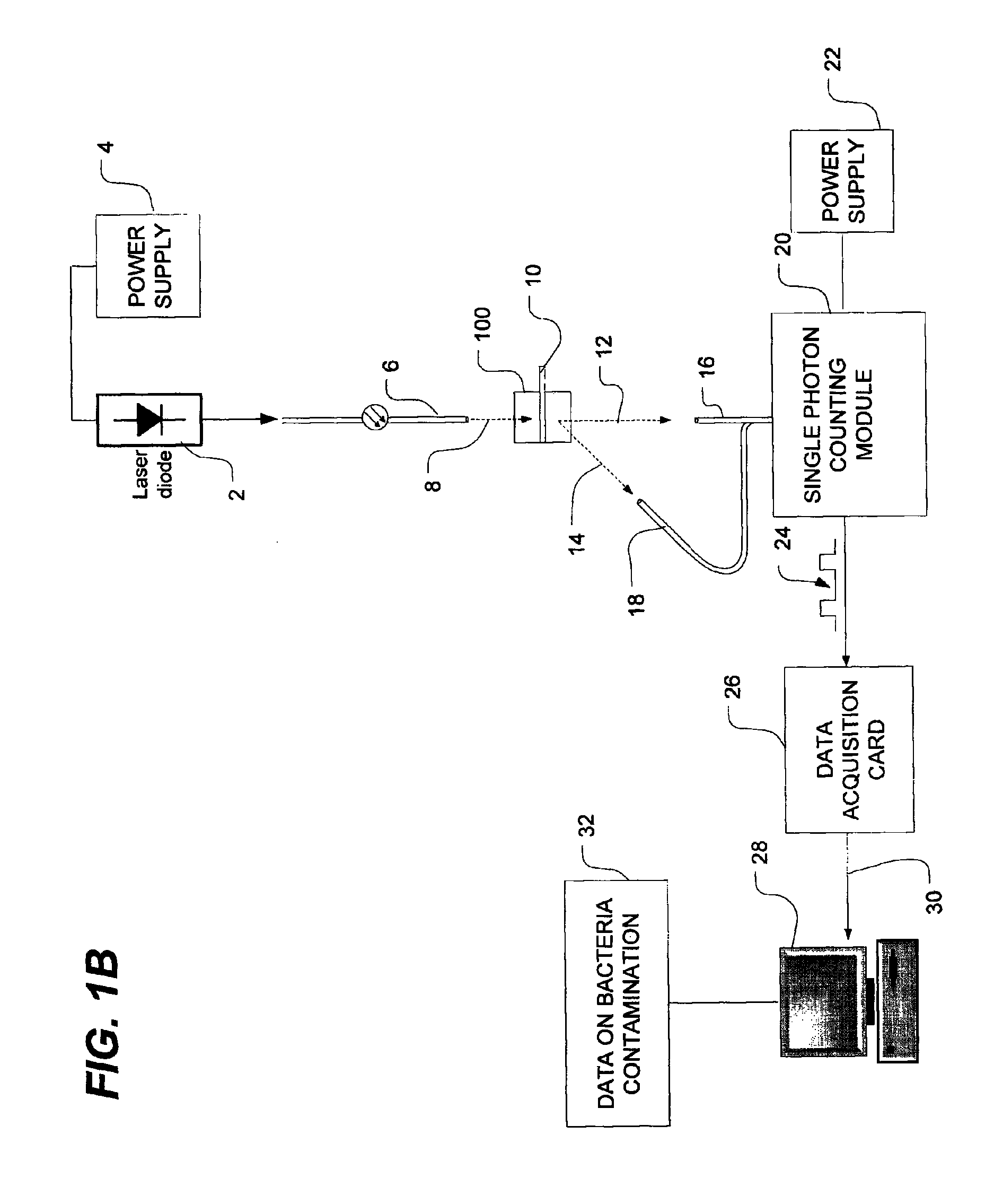
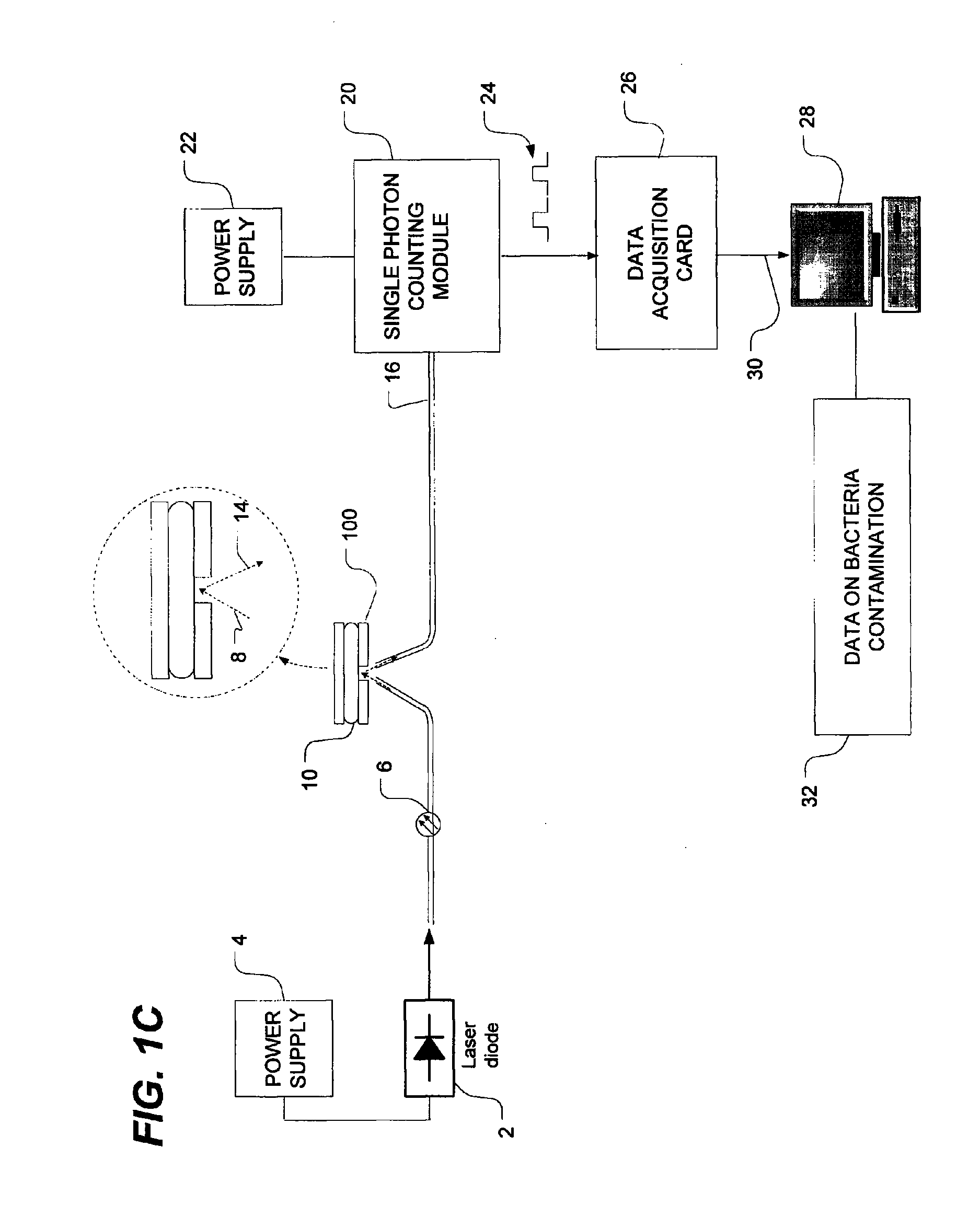
Method of detecting bacterial contamination using dynamic light scattering
InactiveUS8877458B2High strengthReduce intensityBioreactor/fermenter combinationsBiological substance pretreatmentsDynamic light scatteringMicroparticle
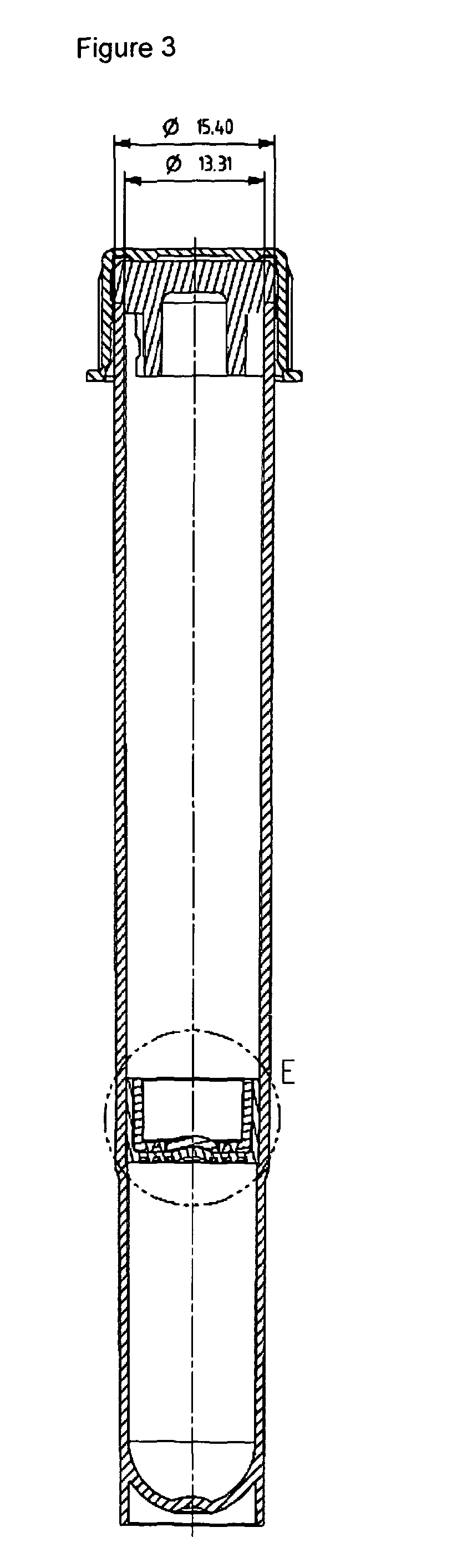
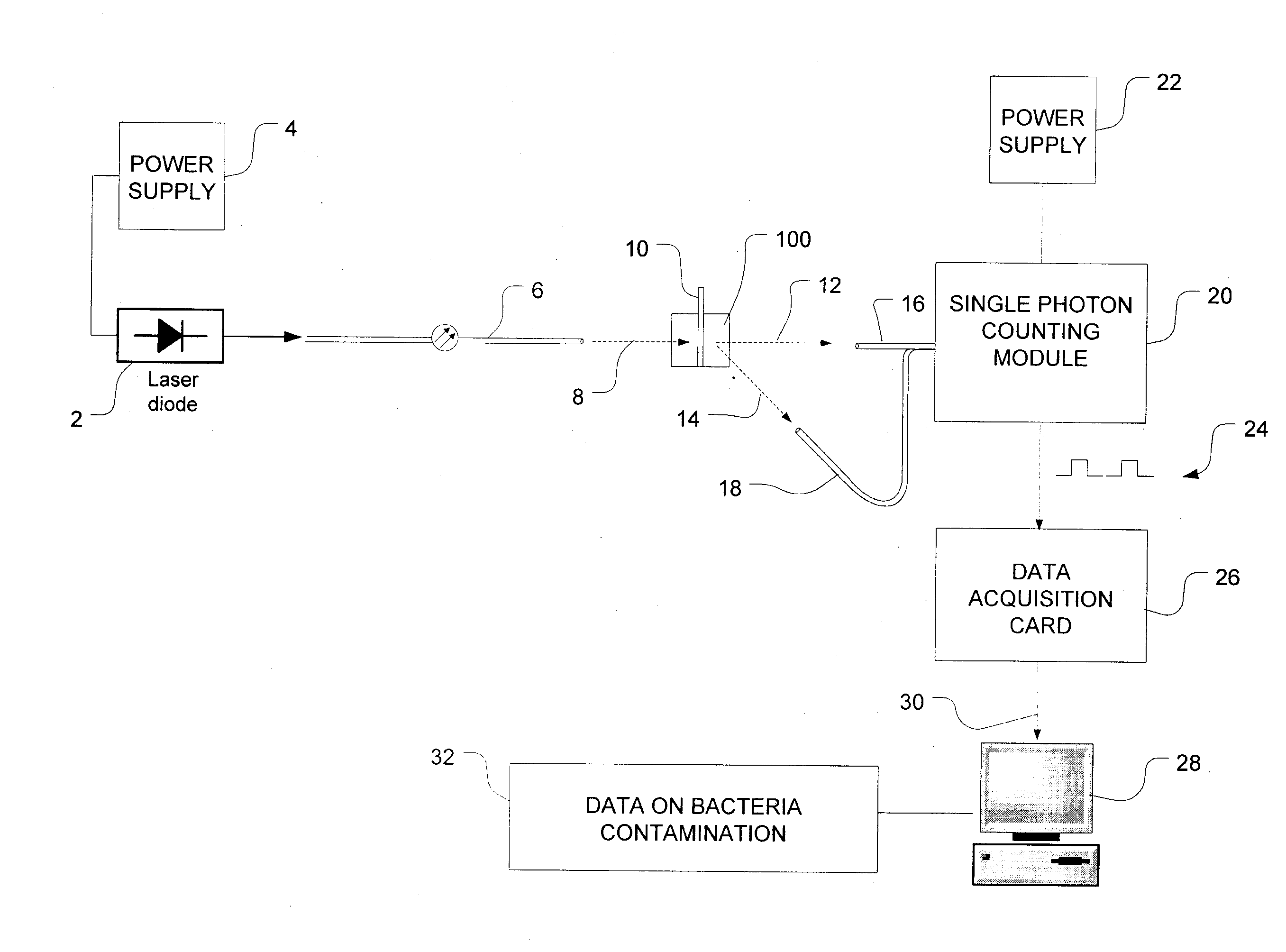
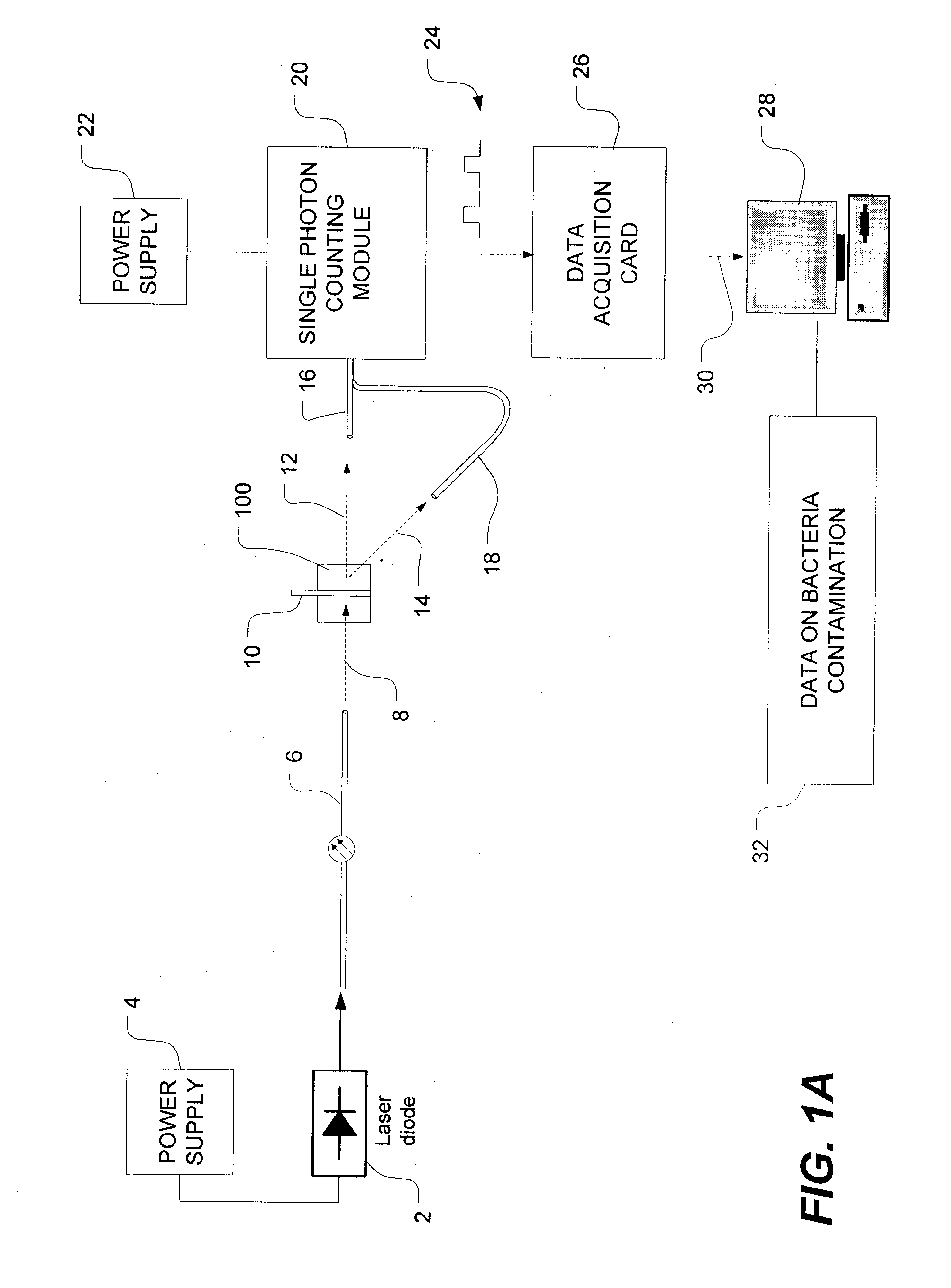
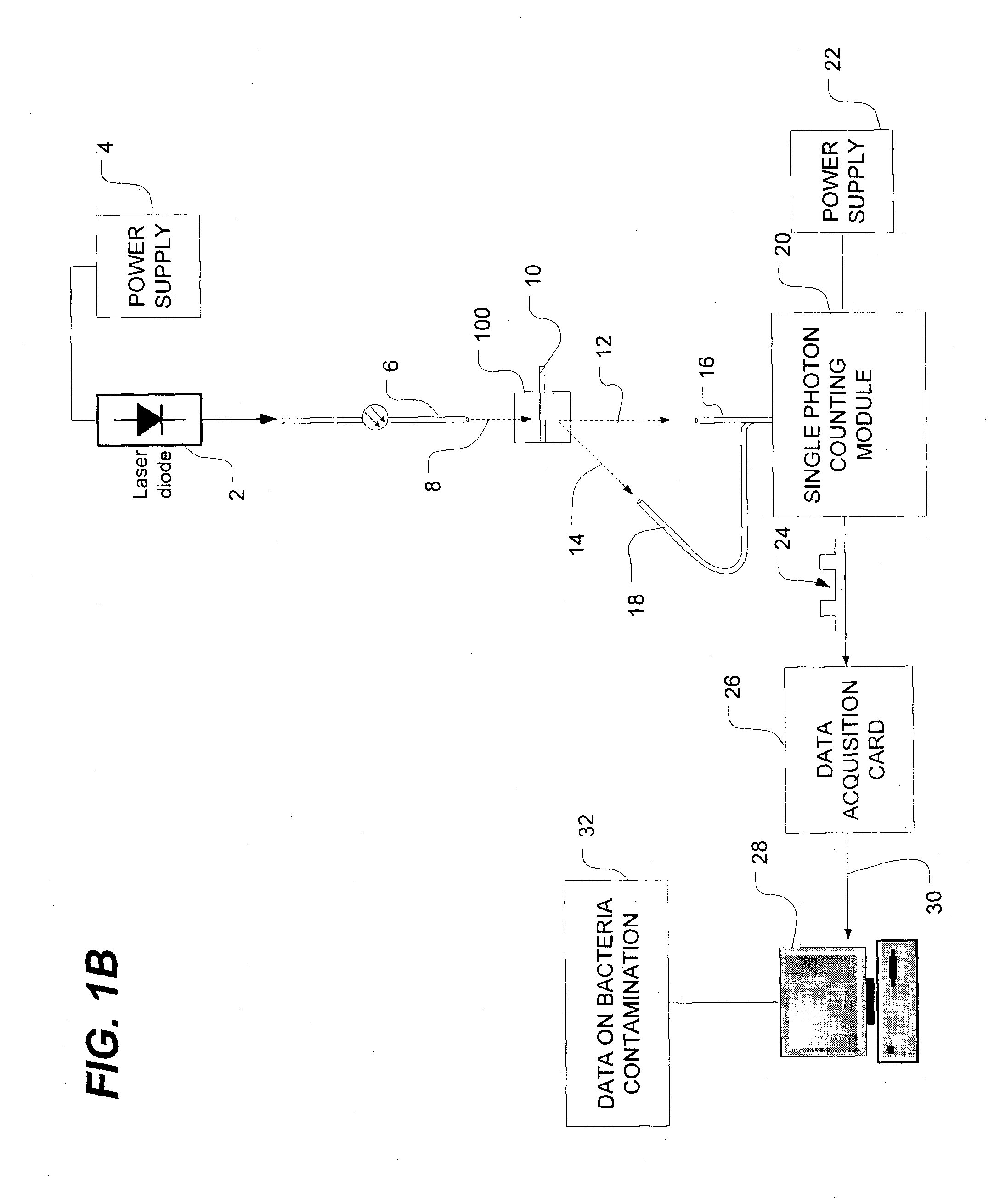
Methods of detecting bacterial contamination in a platelet concentrate are performed using a dynamic light scattering (DLS) instrument and a sample holder. A sample of platelet concentrate can be held vertically or horizontally in a capillary in the sample holder. Alternatively, novel platelet storage bags modified to include an optically translucent window can be held within another variant of the sample holder. Still alternatively, platelet storage bags having one or more tubes detachably appended to the bag can be used. A sample is drawn off into an appended tube for placement directly into the sample holder. This method provides a number of related, non-invasive techniques for detecting whether bacteria has contaminated a platelet concentrate. Contamination indicators include a population of particles different from platelets, microparticles or proteins, bad-quality platelets, i.e. low DLS score, and very high or very low scattering intensity.
Owner:CANADIAN BLOOD SERVICES
Platelet suspensions and methods for resuspending platelets
InactiveUS6326197B1Dead animal preservationMammal material medical ingredientsBiochemistrySalt solution
Platelet suspensions and methods for resuspending platelet concentrates are disclosed. The platelet concentrates are resuspended by combining a platelet concentrate with a substance capable of resuspending platelets, such as a salt solution. The resuspended platelets may be stored and / or administered to a patient.
Owner:FENWAL
Process, tube and device for the preparation of wound healant composition
ActiveUS20130058906A1Increase concentrationCosmetic preparationsOrganic active ingredientsMedicineThrombin activity
Owner:REGENLAB USA LLC
Enhancers for microbiological disinfection
InactiveUS6881731B1Increase powerEnhanced inhibitory effectAntibacterial agentsBiocideDisinfectantOrganic dye
Simple carboxylic acids, in particular dicarboxylic acids such as citric acid shows an unexpected ability to enhance the antimicrobial power of a wide range of disinfectant and / or antibiotic agents. As little as 1% citrate greatly enhances the ability of antibiotics to kill or inhibit a wide range of bacterial species including antibiotic resistant strains. Citrate alone is effective in preventing bacterial growth in platelet concentrates and in red blood cell suspensions. Effective concentrations of citrate cause little if any damage to blood cells. Besides enhancing the power of antibiotics citrate also enhances the antimicrobial properties of disinfectant organic dyes such as crystal violet and methylene blue. In addition citrate enhances the antimicrobial properties of polyphenols of plant origin. Iodine-based disinfectants are also enhanced without enhancing protein denaturation.
Owner:SHANBROM TECH
Method of detecting bacterial contamination using dynamic light scattering
InactiveUS20100136611A1Increased scattering intensityReduce the scattering intensityBioreactor/fermenter combinationsBiological substance pretreatmentsDynamic light scatteringMicroparticle
Methods of detecting bacterial contamination in a platelet concentrate are performed using a dynamic light scattering (DLS) instrument and a sample holder. A sample of platelet concentrate can be held vertically or horizontally in a capillary in the sample holder. Alternatively, novel platelet storage bags modified to include an optically translucent window can be held within another variant of the sample holder. Still alternatively, platelet storage bags having one or more tubes detachably appended to the bag can be used. A sample is drawn off into an appended tube for placement directly into the sample holder. This method provides a number of related, non-invasive techniques for detecting whether bacteria has contaminated a platelet concentrate. Contamination indicators include a population of particles different from platelets, microparticles or proteins, bad-quality platelets, i.e. low DLS score, and very high or very low scattering intensity.
Owner:CANADIAN BLOOD SERVICES
Process for preparing an autologous platelet gel and membrane thereof
A process is disclosed for preparing an autologous platelet gel and membranes thereof comprising mixing a platelet concentrate with a calcium salt and batroxobin. This process encompassing the use of batroxobin as the gel activator allows to overcome the prior art processes drawbacks connected with the use for the same purpose of human of bovine thrombin. A kit is also described for carrying out this process.
Owner:SACCHI MARIA CRISTINA
Automated methods and systems for providing platelet concentrates with reduced residual plasma volumes and storage media for such platelet concentrates
Automated systems and methods for providing platelet concentrates and synthetic storage media with reduced residual plasma volumes are disclosed. The disclosed systems and methods reduce the residual volume of plasma in platelet concentrate to obtain a platelet product having a volume of plasma that is approximately 5% or less of the total platelet product volume. The disclosed systems and methods also reduce the residual volume of plasma in platelet concentrate to obtain a washed platelet product, wherein the volume of plasma in the washed platelet product is approximately 1% or less of the total washed platelet product volume. Storage media for platelets including less than approximately 10% plasma are also disclosed.
Owner:FENWAL
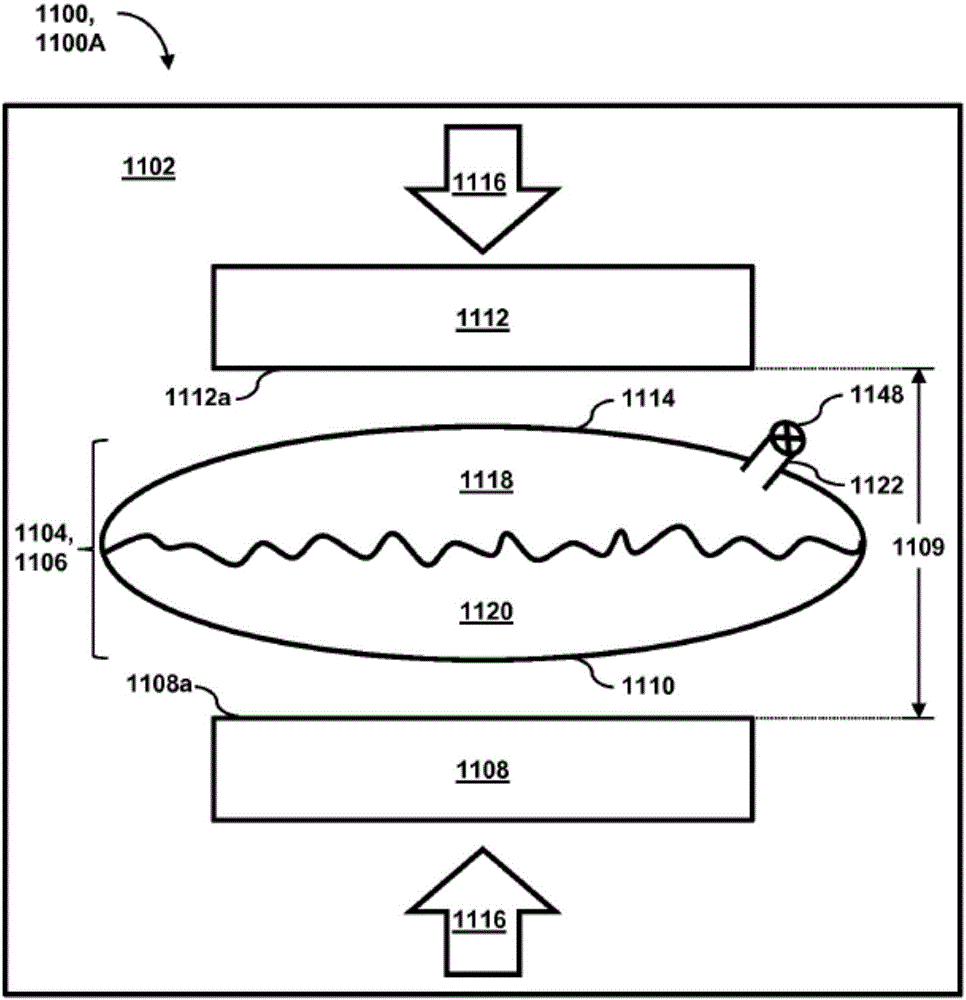
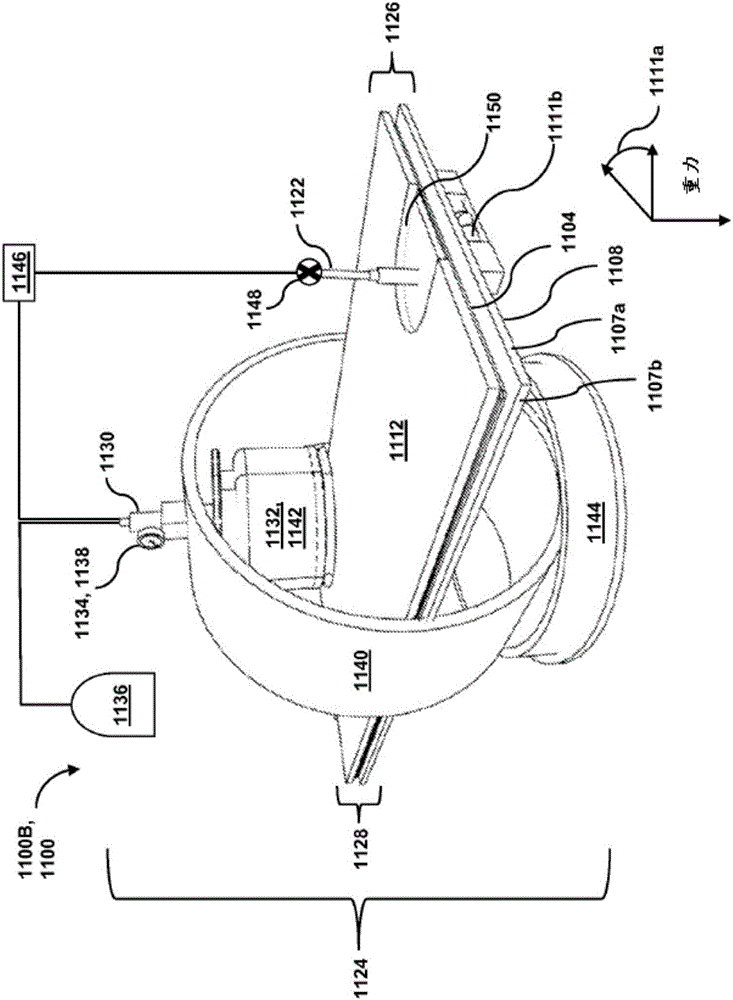
Passive separation of whole blood
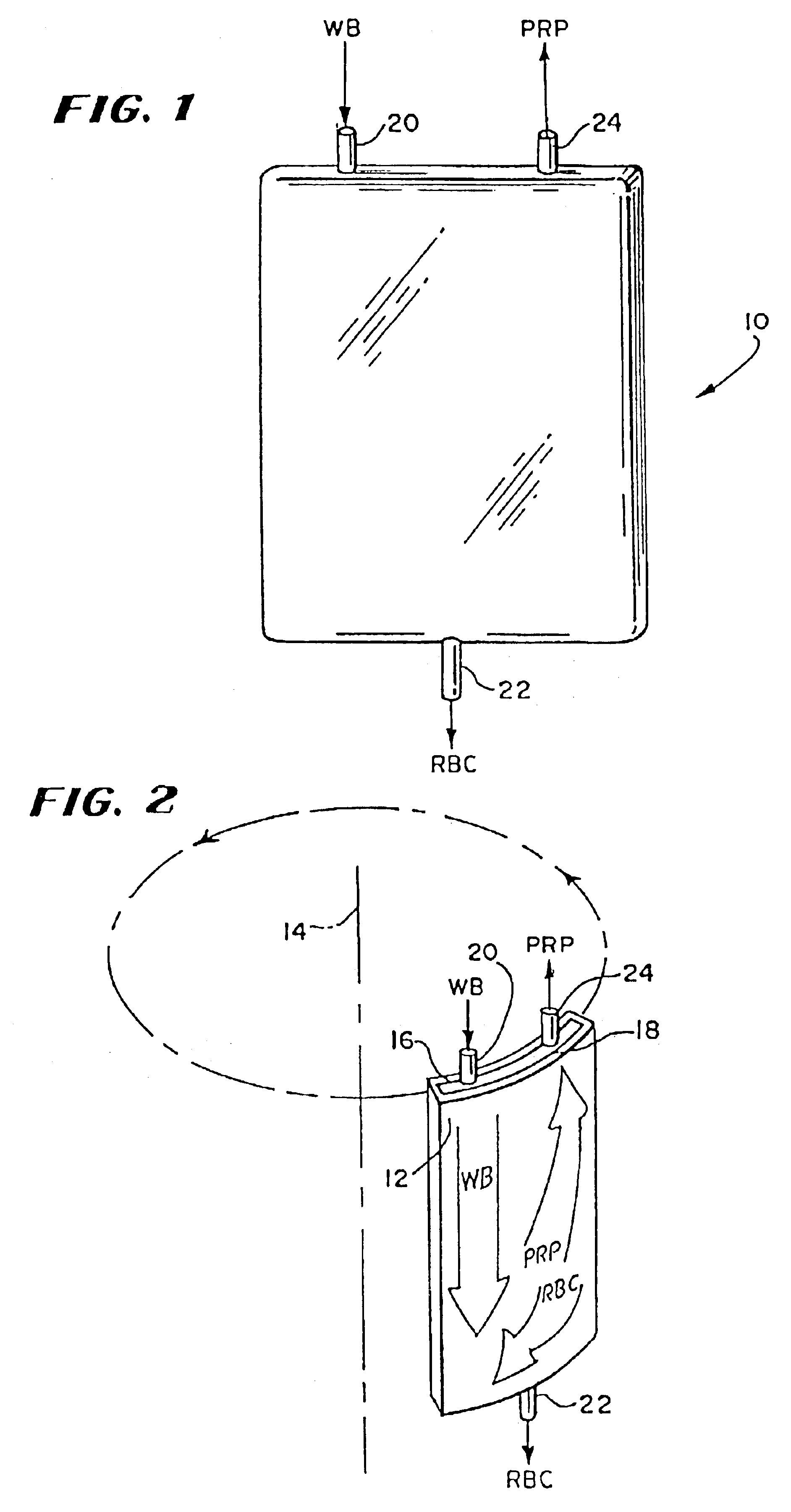
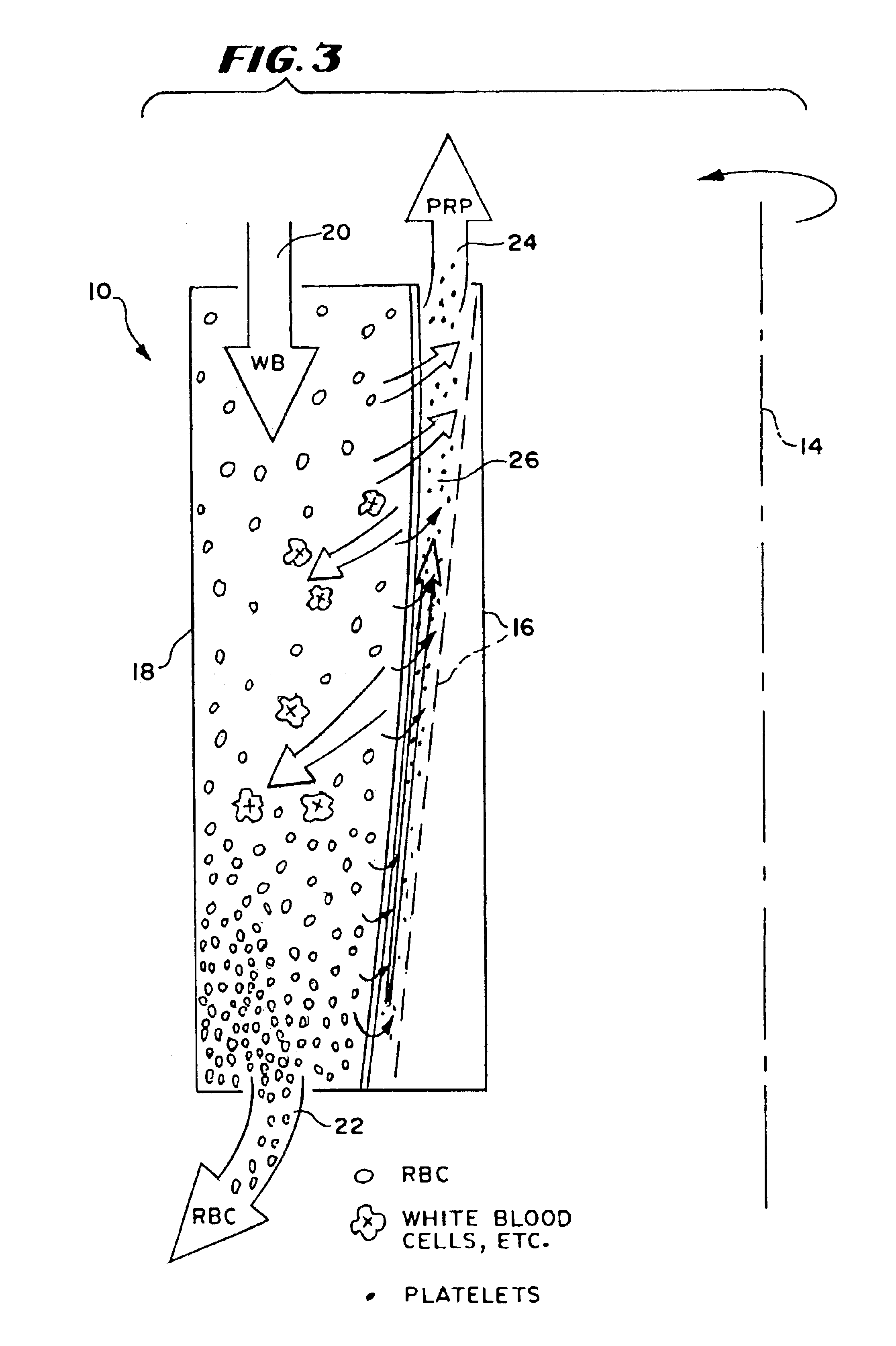
Described are systems, methods, and kits for compression sedimentation and whole blood separation. For example, a compression sedimentation system may include a compression stage configured to accept a flexible reservoir configured to contain a liquid mixture. The compression stage may include a base substrate and a compression substrate configured to apply a force to the flexible reservoir effective to create a pressure in the liquid mixture. An apparatus for whole blood separation may include a sedimentation system that separates whole blood into a supernatant including platelet rich plasma and a subnatant including red blood cells. At least one platelet-concentrating device may be included to receive the supernatant including the PRP and to separate a platelet concentrate and a platelet poor plasma from the supernatant.
Owner:HALCYON BIOMEDICAL
Clottable concentrate of platelet growth factors and preparation method thereof
InactiveUS20110027257A1Improve the level ofCosmetic preparationsPeptide/protein ingredientsCTGFSolvent
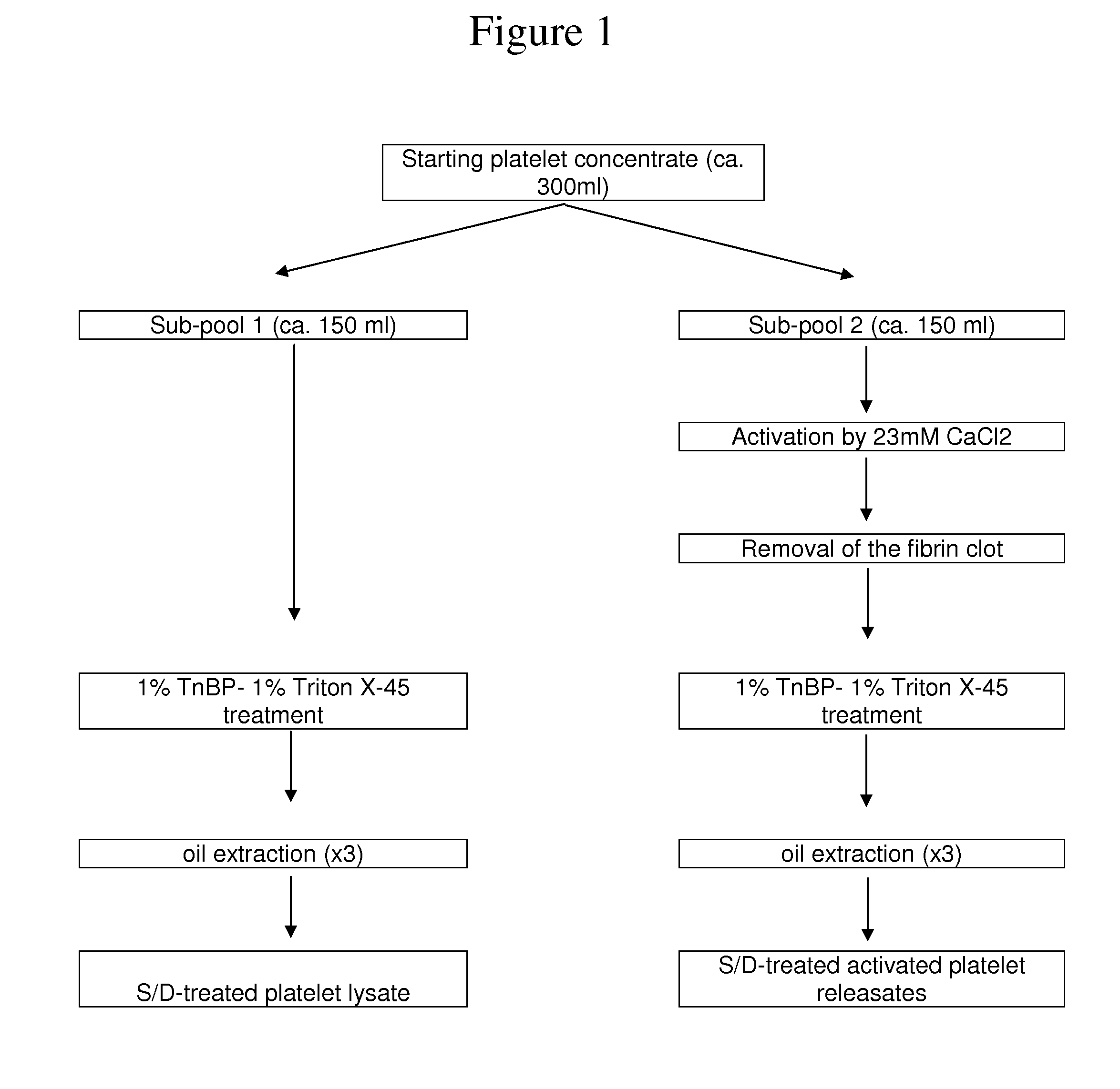
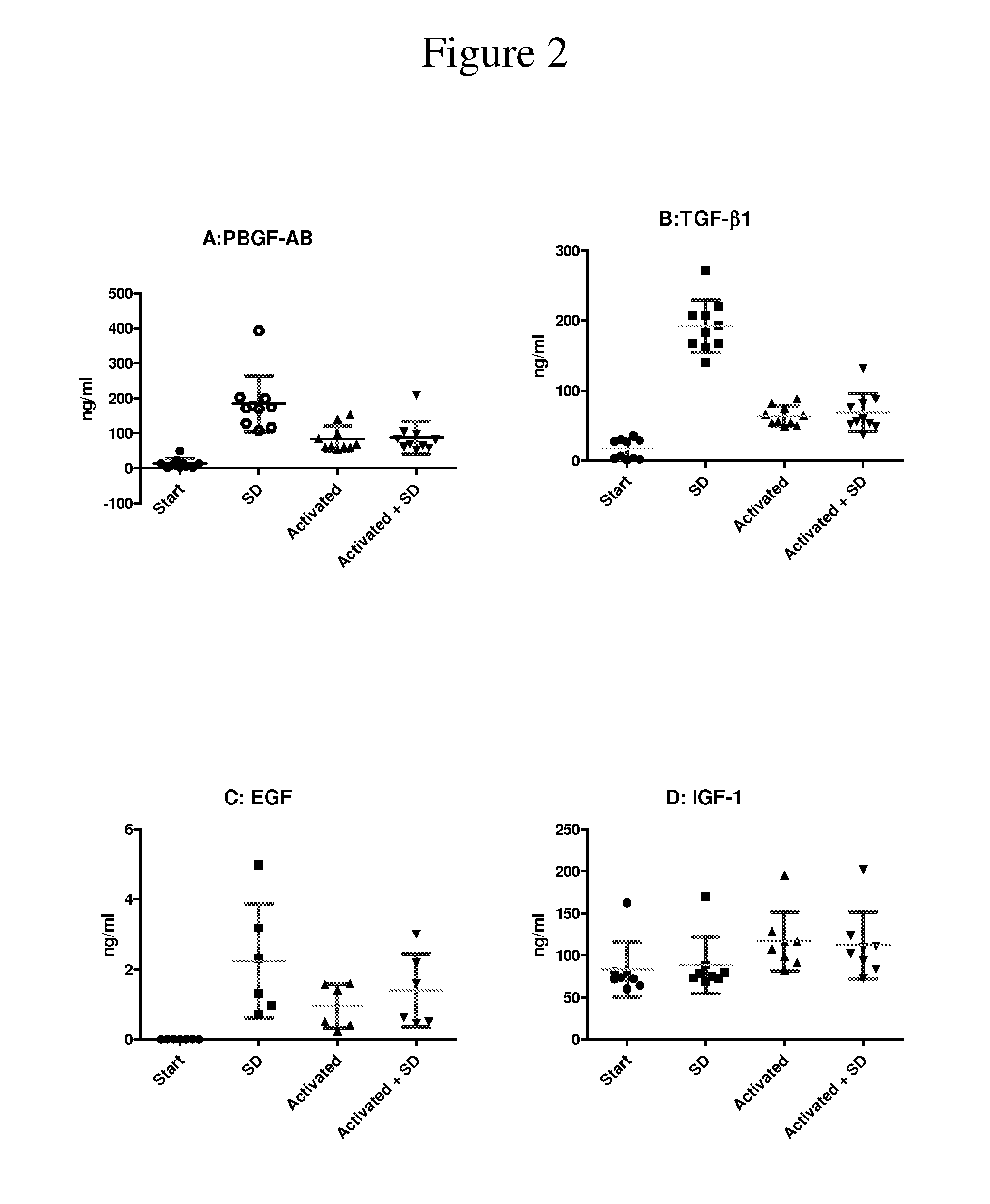
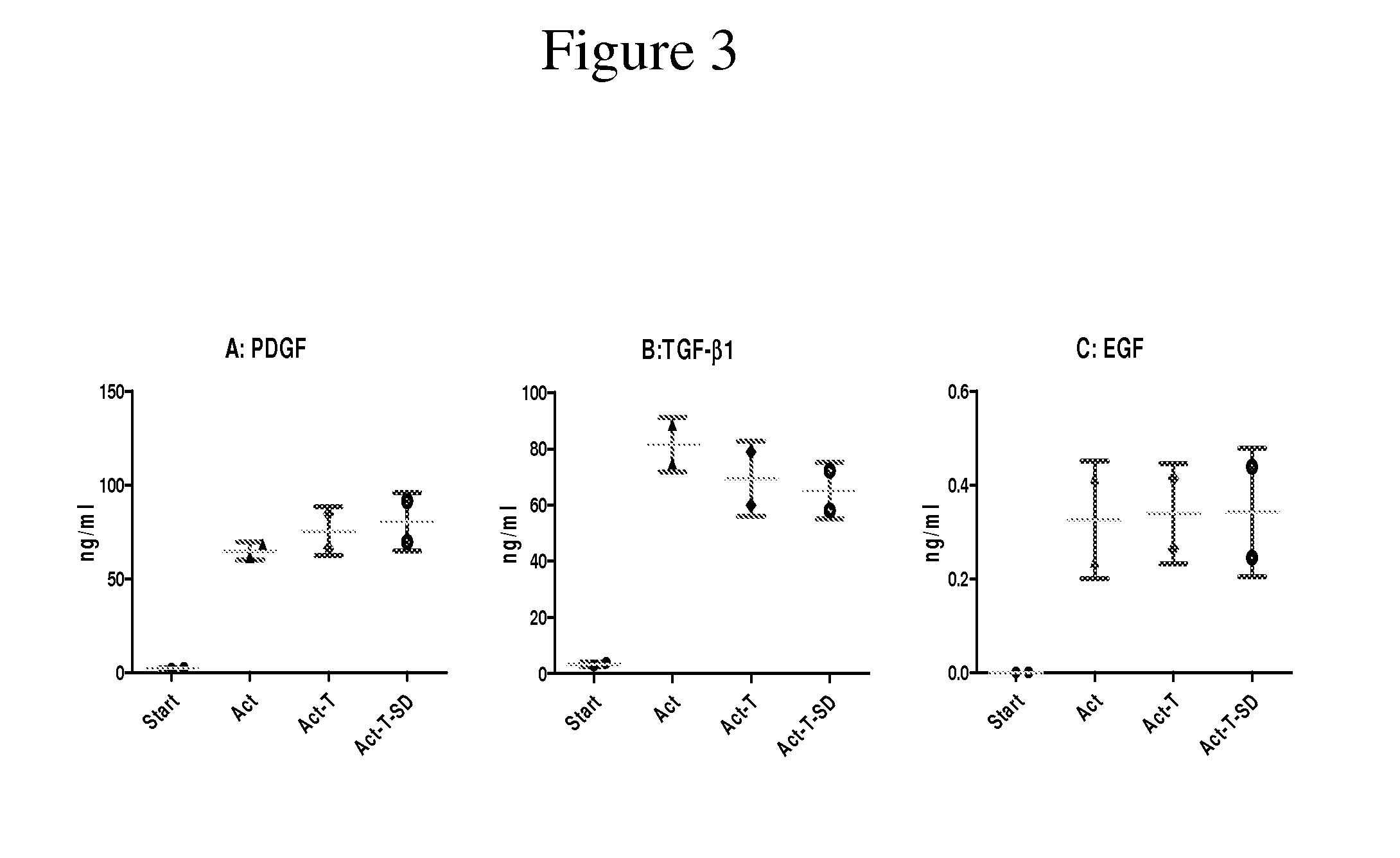
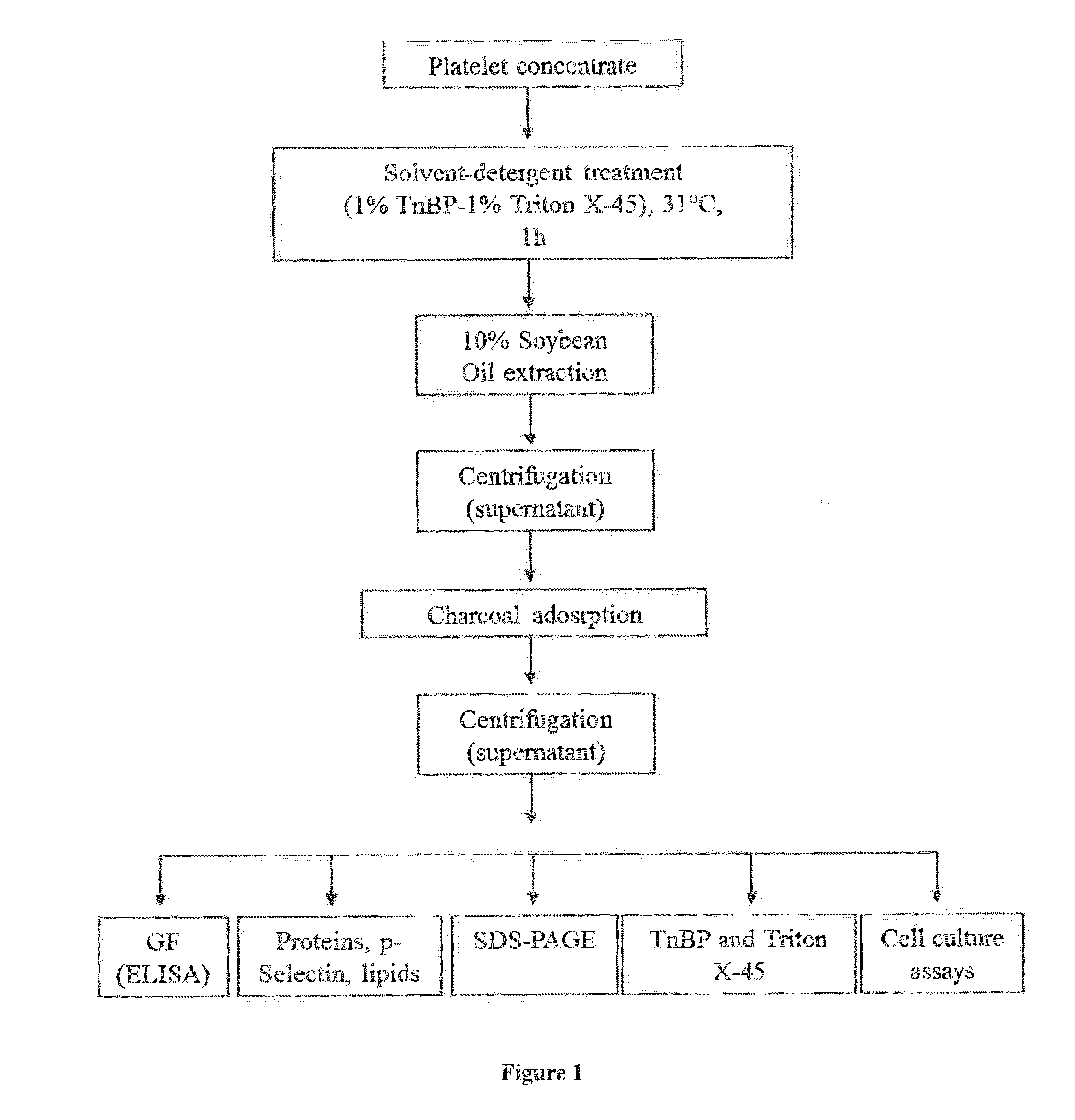
The present disclosure relates to a clottable concentrate of platelet growth factors for therapeutic and / or cosmetic use, preferably comprising the growth factors PDGF, TGT-β, IGF, EGF, CTGF, bFGF and VEGF. In a preferred embodiment, the clottable concentrate of platelet growth factors does not induce blood cell-related transfusion reactions. The present disclosure also relates to a method for preparing a clottable concentrate of platelet growth factors including the steps of contacting a platelet concentrate with a solvent and / or a detergent, incubating the platelet concentrate with the solvent and / or detergent for a period of at least 5 minutes to 6 hours, at a pH maintained in a range from about 6.0 to about 9.0, and at a temperature within the range of from 2° C. to 50° C., preferably within the range of from 25° C. to 45° C., and removing the solvent and / or the detergent by oil extraction and / or chromatographic means.
Owner:ZHENG YANG BIOMEDICAL TECH
Method of preserving a platelet concentrate under elevated xenon concentration and pressure with refrigeration
ActiveUS8158339B2Reducing or eliminating the atmosphere comprising xenonBiocideDead animal preservationRefrigerationXenon
Provides are improved methods for storing platelets and compositions that contain stored platelets for use in transfusions. The method entails obtaining a platelet concentrate from blood obtained from an individual and holding the platelet concentrate in at refrigerated temperatures under an atmosphere having a pressure of from 3.5 to 5 bars comprising more than 65% xenon and for at least one week. Also provided is a refrigerated composition that contains a platelet concentrate, wherein the platelet concentrate contains xenon, and wherein the platelet concentrate has been isolated from an individual for at least seven days.
Owner:RICH TECH HLDG CO LLC
Clottable Concentrate Of Platelet Growth Factors And Preparation Method Thereof
Owner:ZHENG YANG BIOMEDICAL TECH
Process, tube and device for the preparation of wound healant composition
ActiveUS8945537B2Increase concentrationOrganic active ingredientsCosmetic preparationsCell extractionBiochemistry
Owner:REGENLAB USA LLC
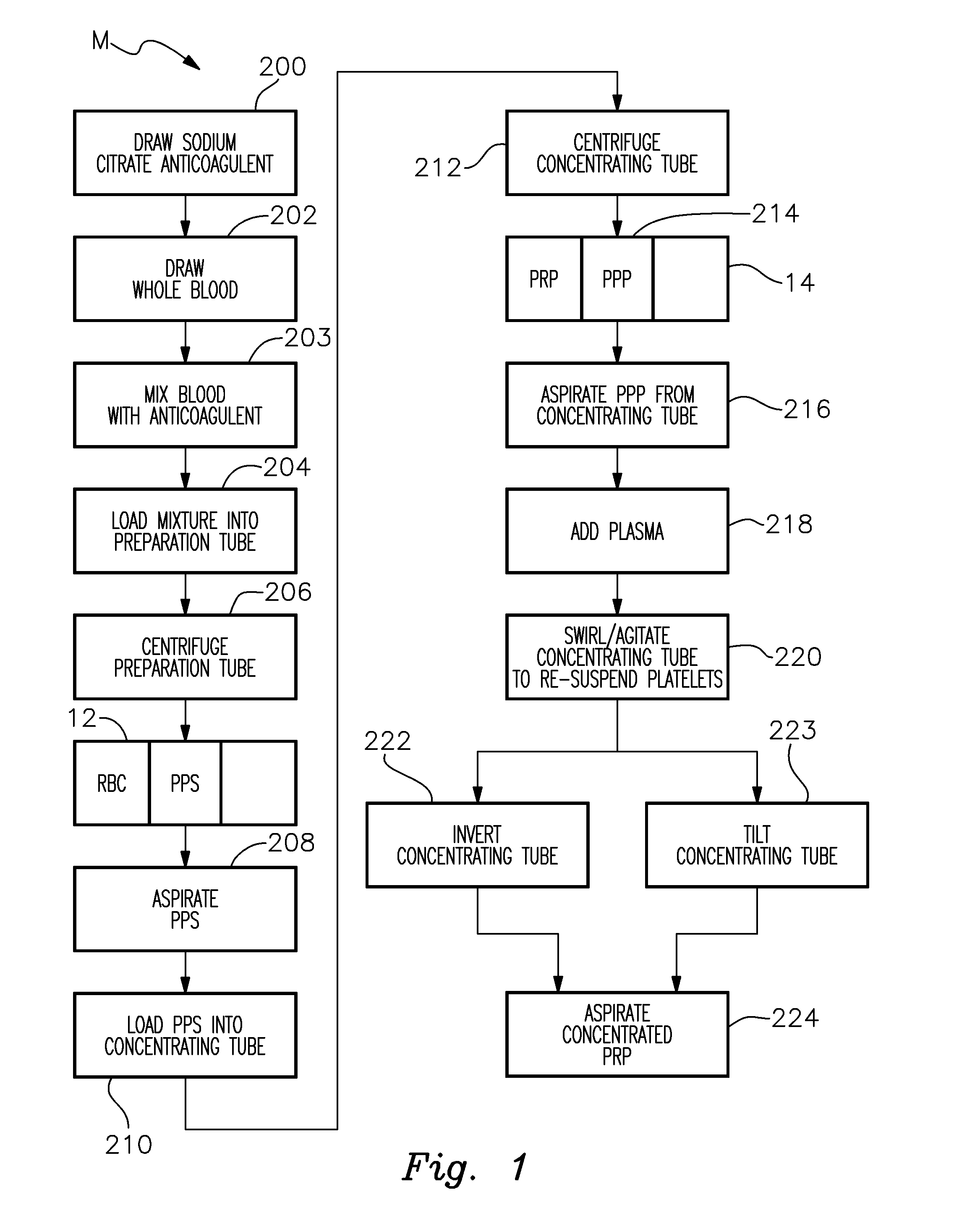
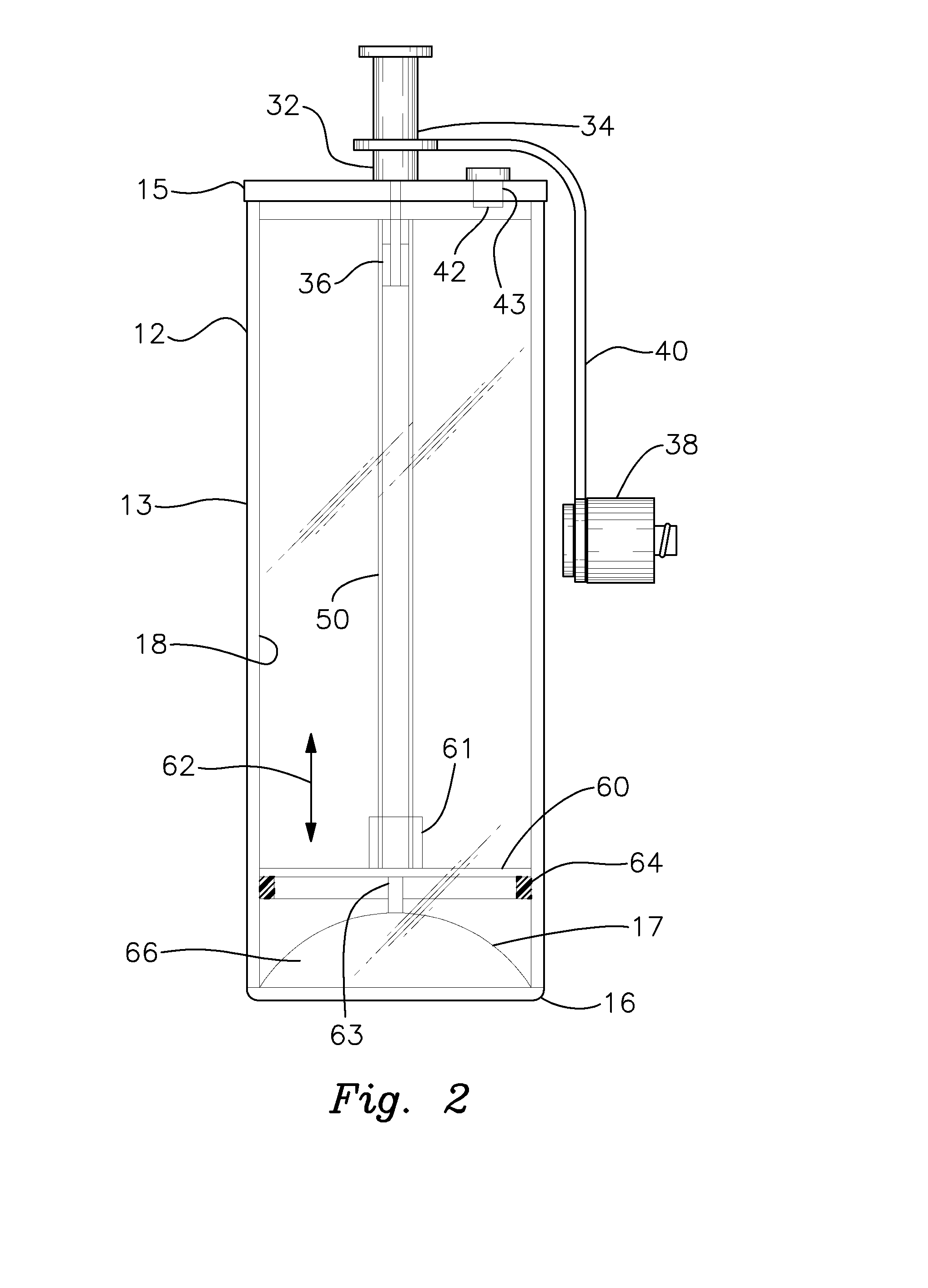
Platelet Concentrating System
InactiveUS20150367064A1Effectively and efficiently manufacturedHighly concentrated and medically effective PRP productOther blood circulation devicesDispersed particle separationMedicinePlatelets blood
A method for more effectively concentrating blood platelets for use in medical procedures includes providing preparation and concentrating tubes. Anticoagulated whole blood is added to the preparation tube, which is centrifuged to separate red blood cells from a platelet plasma suspension The platelet plasma suspension is aspirated and loaded into the concentrating tube, which is itself centrifuged to separate the platelet plasma suspension into platelet poor plasma and platelet rich plasma layers. The platelet poor plasma is aspirated through a port of the concentrating tube and the remaining concentrated PRP is aspirated through the same or a different port of the concentrating tube.
Owner:PENNIE PATRICK
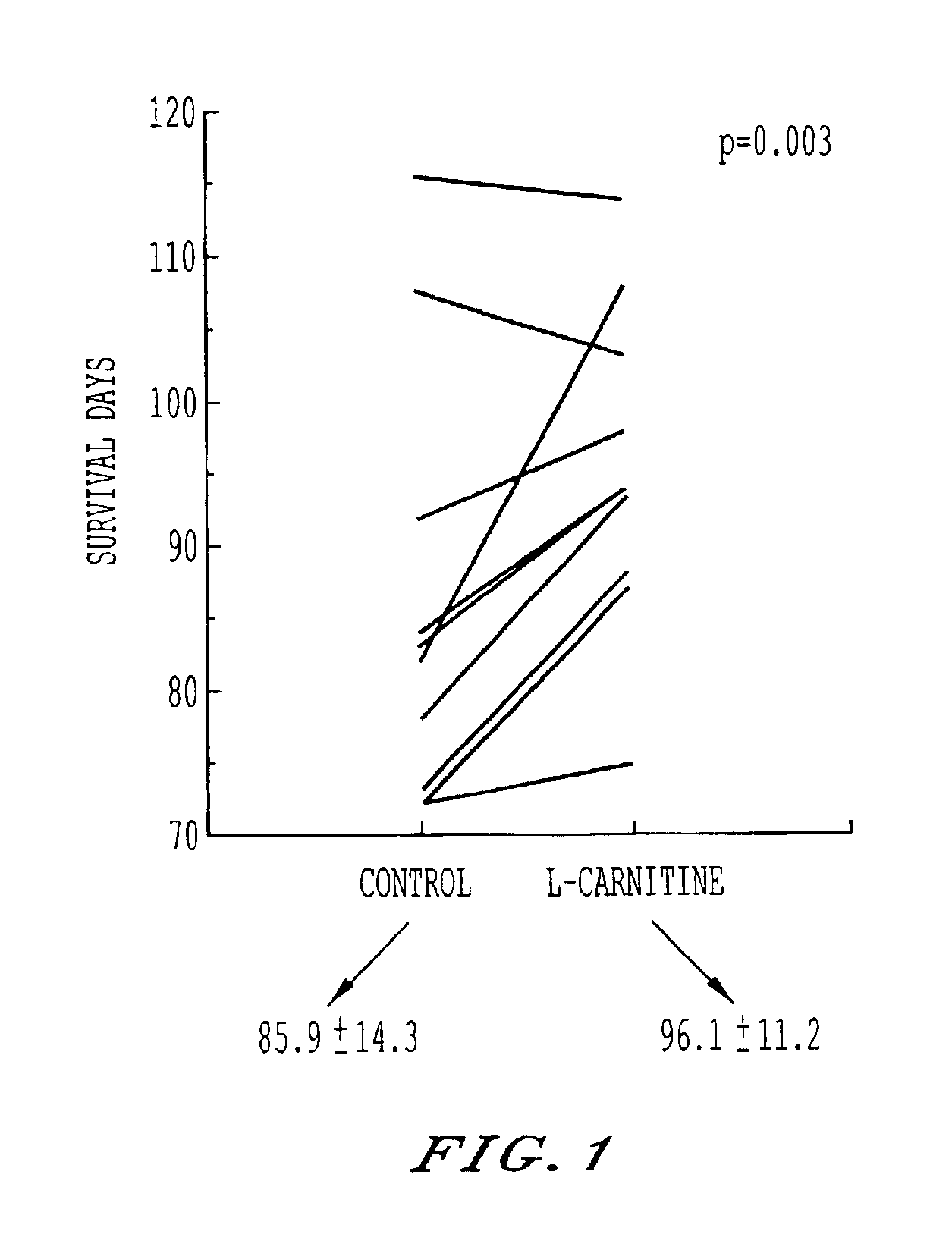
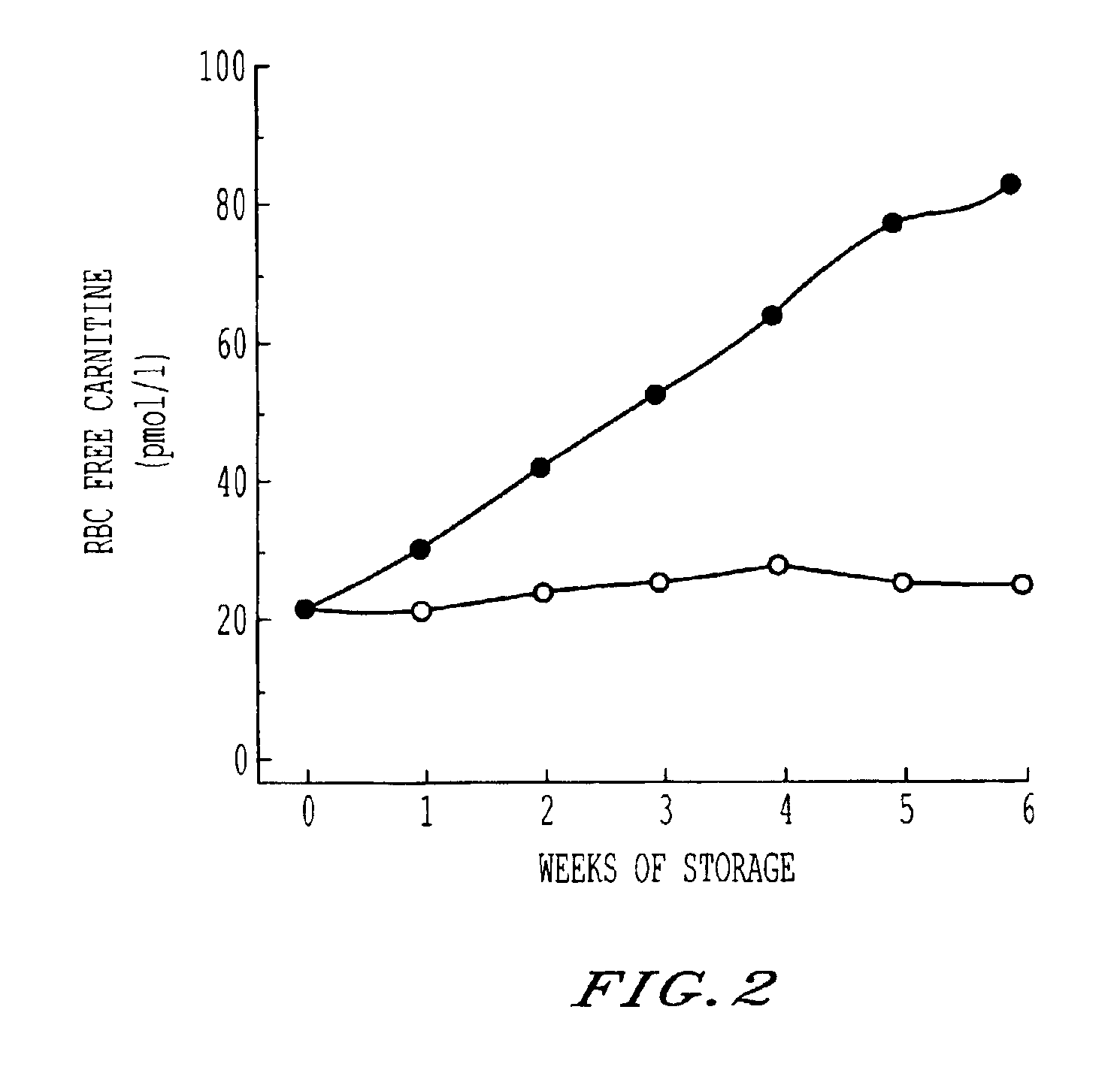
Storage and maintenance of platelets
InactiveUS6960428B2Experience damageOrganic active ingredientsMicrobiological testing/measurementWhole blood productWhite blood cell
Cell membrane maintenance of red blood cells and platelet concentrates is improved by the addition of 1 mM-10 mM L-carnitine and derivatives. This improvement allows extension of the period of viability of packed red blood cells and platelet concentrations beyond current periods. Additionally, the materials so treated exhibit extended circulation half life upon transfusion to a patient. Improvements in membrane maintenance achieved by this method permit irradiation of sealed containers of blood products so as to substantially sterilize and destroy leukocytes in the same.
Owner:SIGMA TAU IND FARMACEUTICHE RIUNITE SPA
Automated methods and systems for providing platelet concentrates with reduced residual plasma volumes and storage media for such platelet concentrates
ActiveUS20140037750A1Plasma-reduced plateletBiocideInorganic phosphorous active ingredientsBlood plasmaWashed platelet
Automated systems and methods for providing platelet concentrates and synthetic storage media with reduced residual plasma volumes are disclosed. The disclosed systems and methods reduce the residual volume of plasma in platelet concentrate to obtain a platelet product having a volume of plasma that is approximately 5% or less of the total platelet product volume. The disclosed systems and methods also reduce the residual volume of plasma in platelet concentrate to obtain a washed platelet product, wherein the volume of plasma in the washed platelet product is approximately 1% or less of the total washed platelet product volume. Storage media for platelets including less than approximately 10% plasma are also disclosed.
Owner:FENWAL
Method and apparatus for producing platelet rich plasma and/or platelet concentrate
InactiveUS20080206858A1Easy to produceSmall amount is easilyBioreactor/fermenter combinationsBiological substance pretreatmentsBlood plasmaRed Cell
Platelet rich plasma and / or platelet concentrate is prepared by placing whole blood in a first chamber of a sterile processing disposable having two chambers. The processing disposable is subjected to a first centrifugation to separate red blood cells, and the resulting platelet rich plasma supernatant is decanted to the second chamber. The processing disposable is subjected to a second centrifugation to concentrate platelets. A volume of the platelet poor plasma supernatant in the second chamber is removed, and the platelets are re-suspended in the remaining plasma. The second chamber may contain anticoagulant to preclude aggregation of the platelets.
Owner:BLASETTI LOU +1
Virally-Inactivated Growth Factors-Containing Platelet Lysate Depleted of PDGF and VEGF and Preparation Method Thereof
The invention concerns human platelet extracts rich in growth factors (PGF) for wound healing and stem cell expansion. Accordingly the subject invention relates to a virally-inactivated growth factors-containing platelet lysate depleted of PDGF and VEGF, which is preferably enriched in TGF, IGF and EGF-rich. The present invention further concerns a method for obtaining a platelet lysate comprising the steps of contacting a starting platelet concentrate with a solvent and / or a detergent, incubating the starting platelet concentrate with the solvent and / or detergent for a period of at least 5 minutes to 6 hours, at a pH maintained in a range from about 6.0 to about 9.0, and at a temperature within the range of from 2° C. to 50° C., optionally removing the solvent and / or the detergent by oil extraction and obtaining an aqueous protein phase, and incubating the solvent and / or detergent-treated platelet concentrate or the aqueous protein phase with charcoal.
Owner:ZHENG YANG BIOMEDICAL TECH
Modern blood banking employing improved cell preservation composition
InactiveUS20060019234A1Inhibition of activationAvoid damageMammal material medical ingredientsDead animal preservationAmino acid bindingBlood plasma
An improved anticoagulant is based on a higher level of citric acid than is usual (at least about 0.2% weight by volume). The higher citrate is combined with an amino acid as a counterion. The amino acid prevents cellular damage often caused by elevated citrate levels. The amino acid citrate mixture also serves to preserve platelet concentrates and platelet rich plasma during room incubation. Not only does the amino acid citrate combination enhance platelet integrity, it completely inhibits the growth of bacteria such as Staphylococcus epidermidis. Collecting blood of plasma into such higher levels of citrate prevents activation of blood proteins so that fractions made from the blood or plasma have superior characteristics.
Owner:SHANBROM TECH
Triple spin, double pool and revolumization process for concentrating platelets and derivative platelet concentrate
InactiveUS20090202981A1Promote resultsWater/sewage treatment by centrifugal separationCentrifugal force sediment separationCentrifugationMedicine
The present invention comprises an improved method of manufacturing platelet concentrate comprising the steps of: performing a first centrifugation of anticoagulated blood, pooling a first platelet rich sample, performing a second centrifugation on the first platelet rich sample whereby the volume of the first platelet rich sample placed in each subsequent centrifuge container is greater than the extracted volume from each previously utilized container, pooling a second platelet rich sample, performing a third centrifugation on the second platelet rich sample, whereby the volume of the second platelet rich sample placed in each subsequent centrifuge container is greater than the previous extracted volume from each previously utilized container, decanting a portion of supernatant overlying a third platelet rich sample, and suspending the third platelet rich sample such as to form a highly concentrated platelet rich formulation at concentrations greater than 8 times baseline and up to 60 times baseline.
Owner:SMITH ASTLEY E
Platelet Concentrate Preservation Method
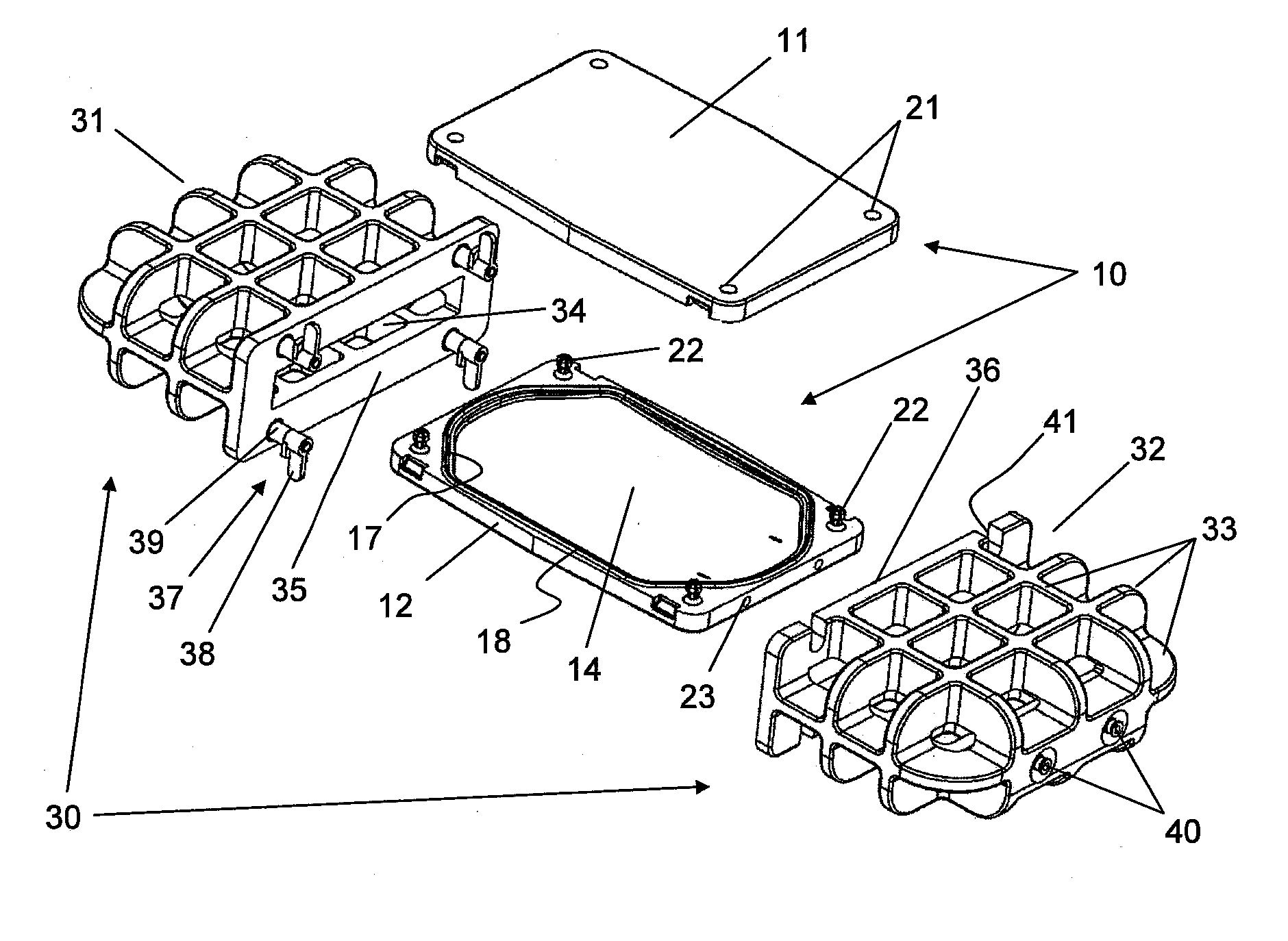
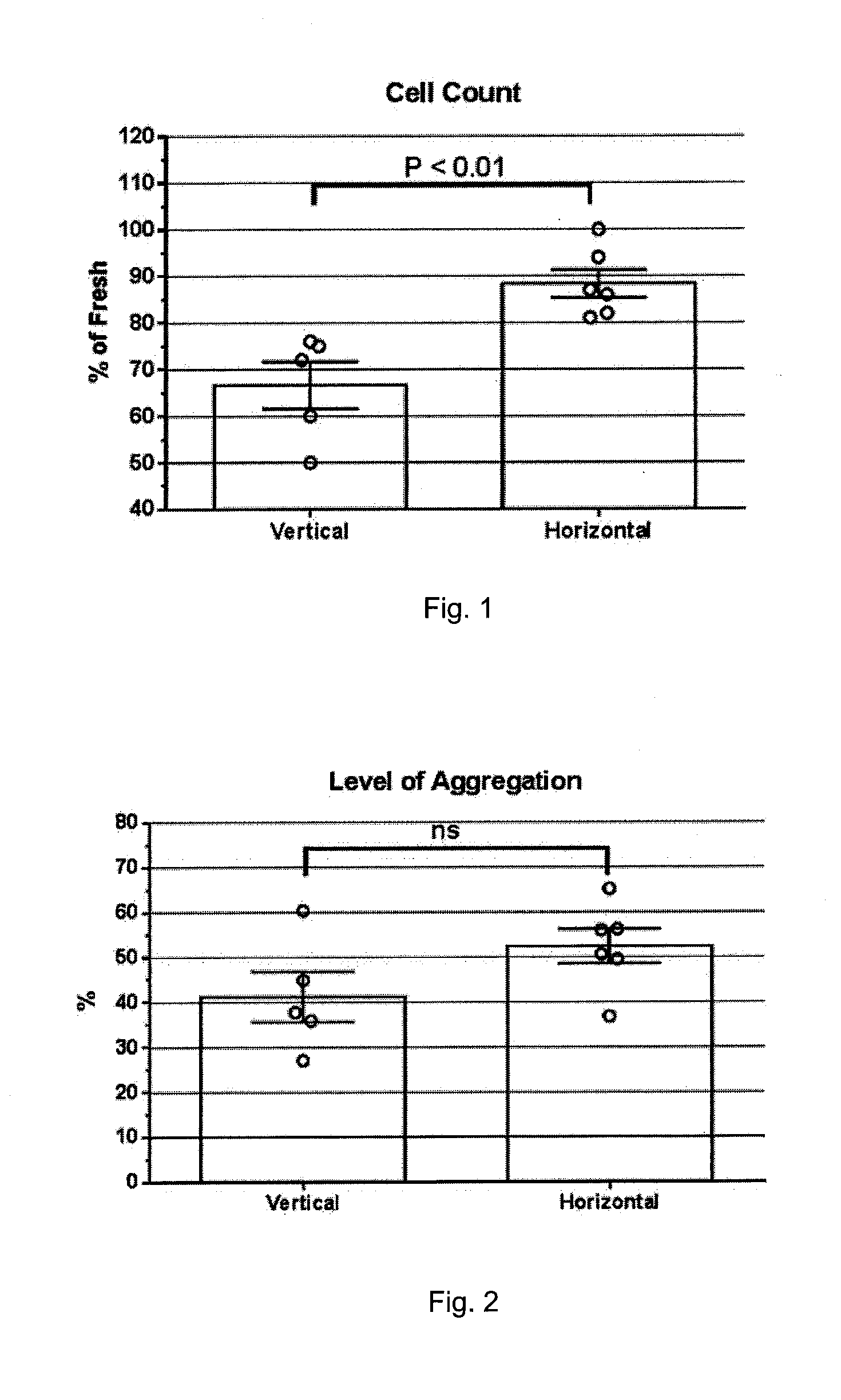
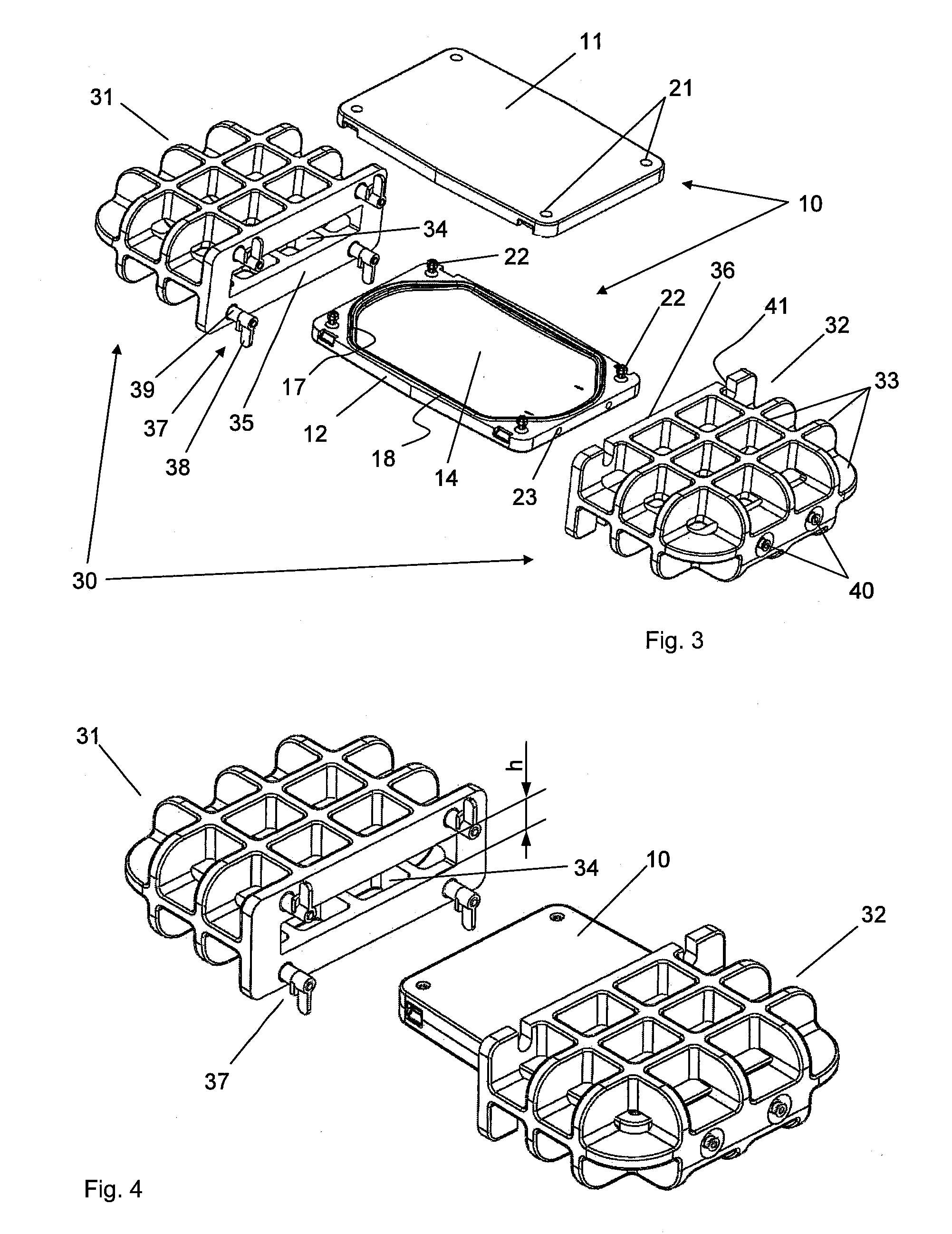
ActiveUS20150305324A1Good disintegrationAvoid generatingPharmaceutical containersMedical devicesWhole blood productHigh intensity
A method and a platelet concentrate preservation device for platelet concentrate storage. A method includes at least partially saturating platelet concentrate xenon, and storing the platelet concentrate at less than 15 C in a generally horizontal position. A device can be used to store blood, blood products, or combinations thereof that may or may not be under pressure. The device includes a chamber having a cavity. The chamber includes first and second chamber parts that form the cavity when releasably connected together. The cavity is designed to receive at least one bag that contains the blood, blood products, or combinations thereof. The device also includes a high-strength casing and includes a chamber cavity. The high-strength casing includes first and second casing parts that form the chamber cavity when releasably connected together. The chamber cavity is designed to receive the chamber.
Owner:RICH TECH HLDG CO LLC +1
Compositions and methods for cell culture
InactiveUS20170175080A1Growth promoting activityIncrease cell densityOrganic active ingredientsCosmetic preparationsFreeze thawingHuman platelet
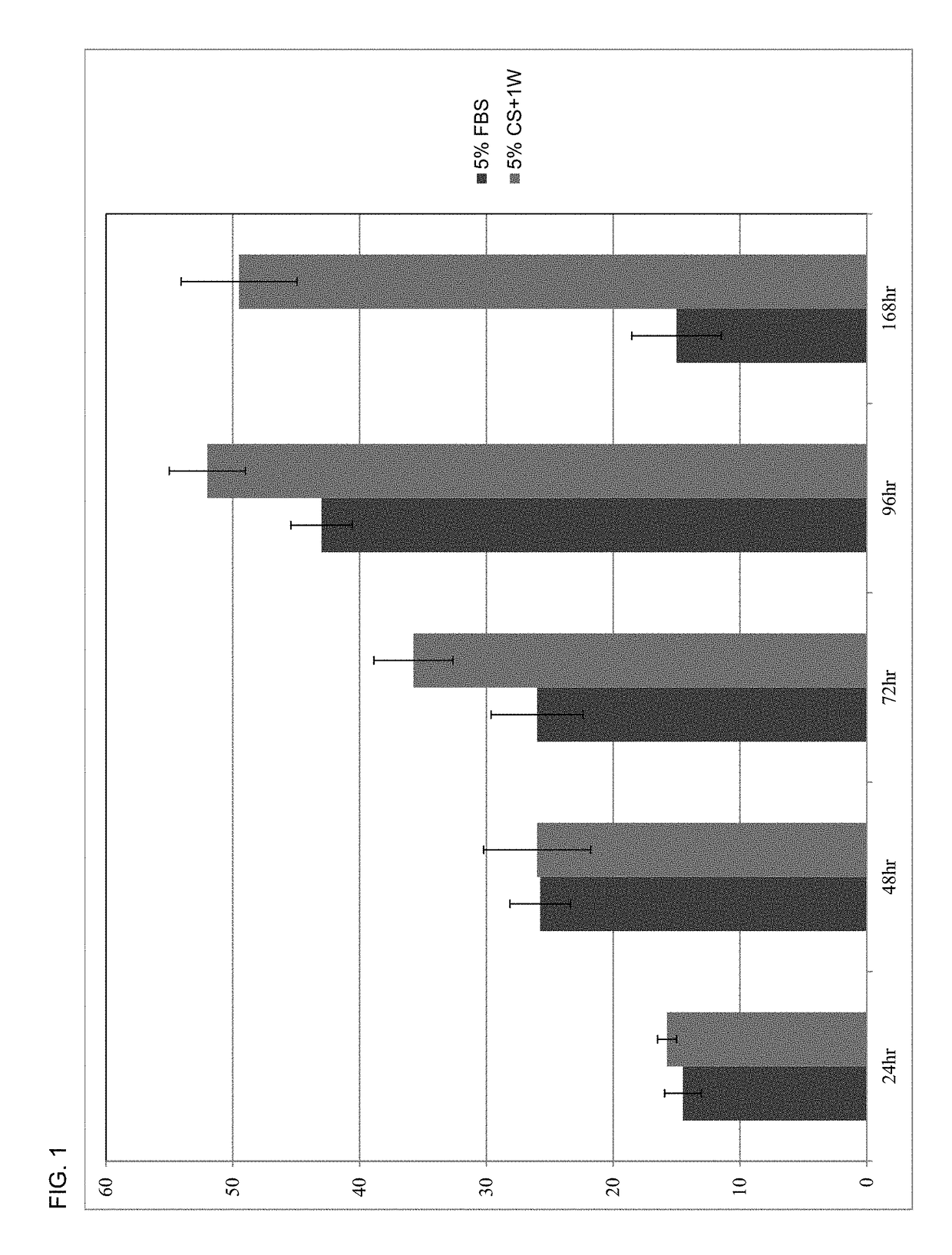
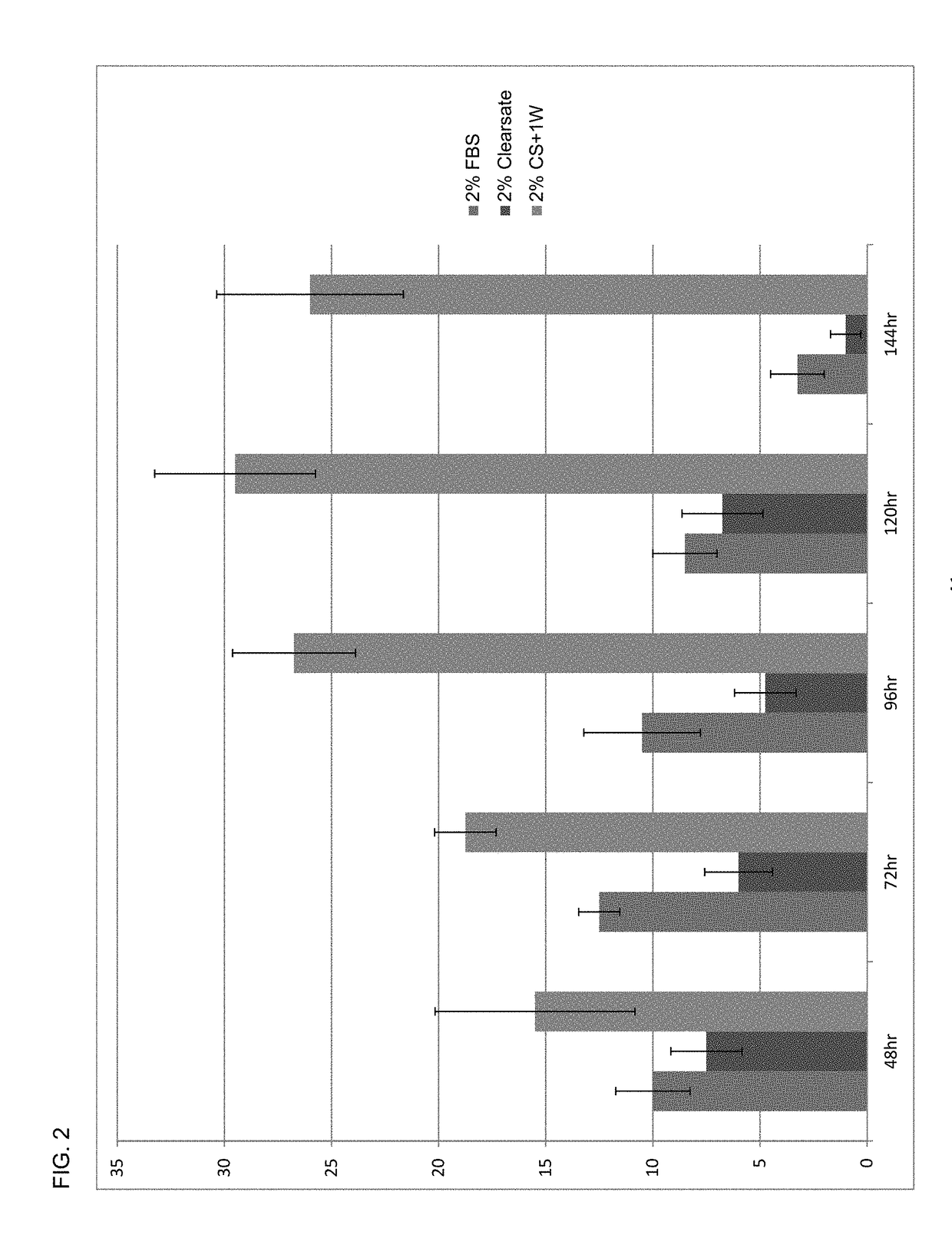
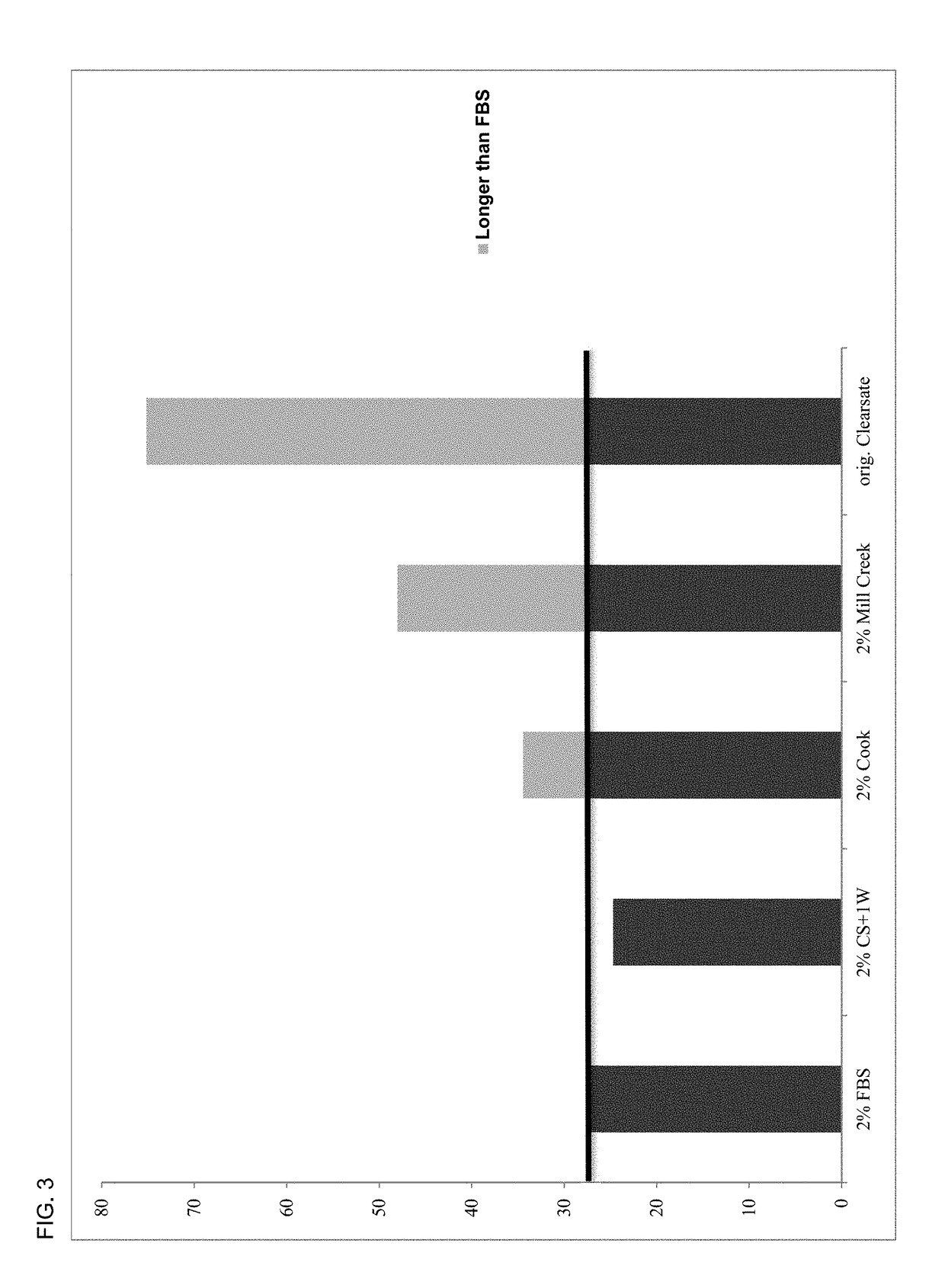
A human platelet lysate preparation that contains human plasma is provided. This preparation can be generated from human platelets by concentrating and washing them under defined conditions to control the degree of human plasma present. The washed platelet concentrate is then subjected to a freeze-thaw cycle to produce platelet lysate which is centrifuged and filtered through 0.65μ, 0.45μ and 0.2μ filters, aliquoted and stored frozen at <−20° C. until thawed for use. This invention describes the novel finding that the controlled addition of human plasma to the lysate preparation significantly enhances the cell growth potency of the lysate preparation. This lysate can be used as a media supplement to replace fetal bovine serum (FBS) or other non-human serum additives used for the culture of mammalian cells. This invention also describes the formulation and use of the lysate preparation as a topical application for skin care, and wound healing, including anti-wrinkling, anti-scarring and wound resolution applications of the invention.
Owner:SAN DIEGO BLOOD BANK
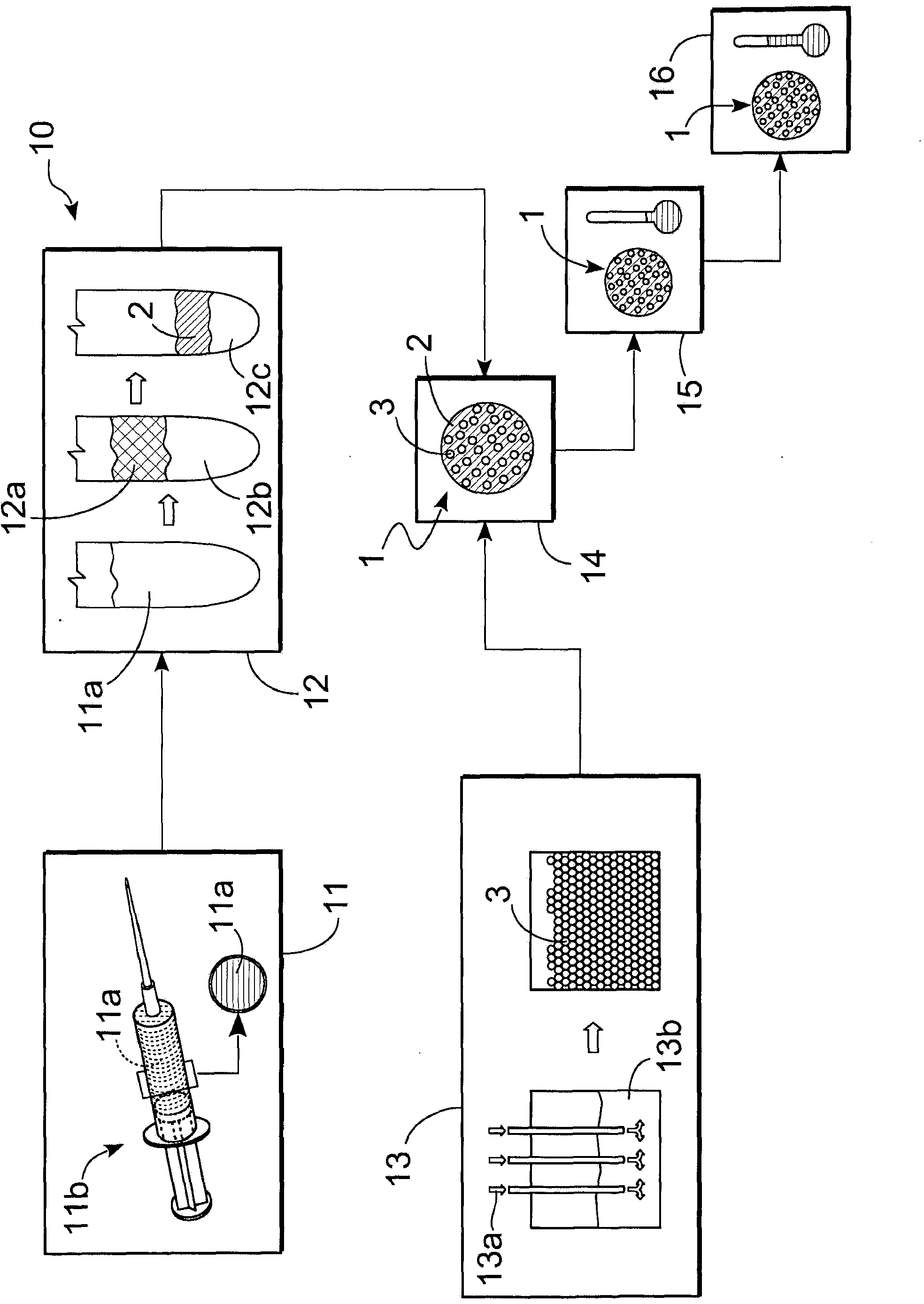
Therapeutic preparation and process for preparing said therapeutic preparation
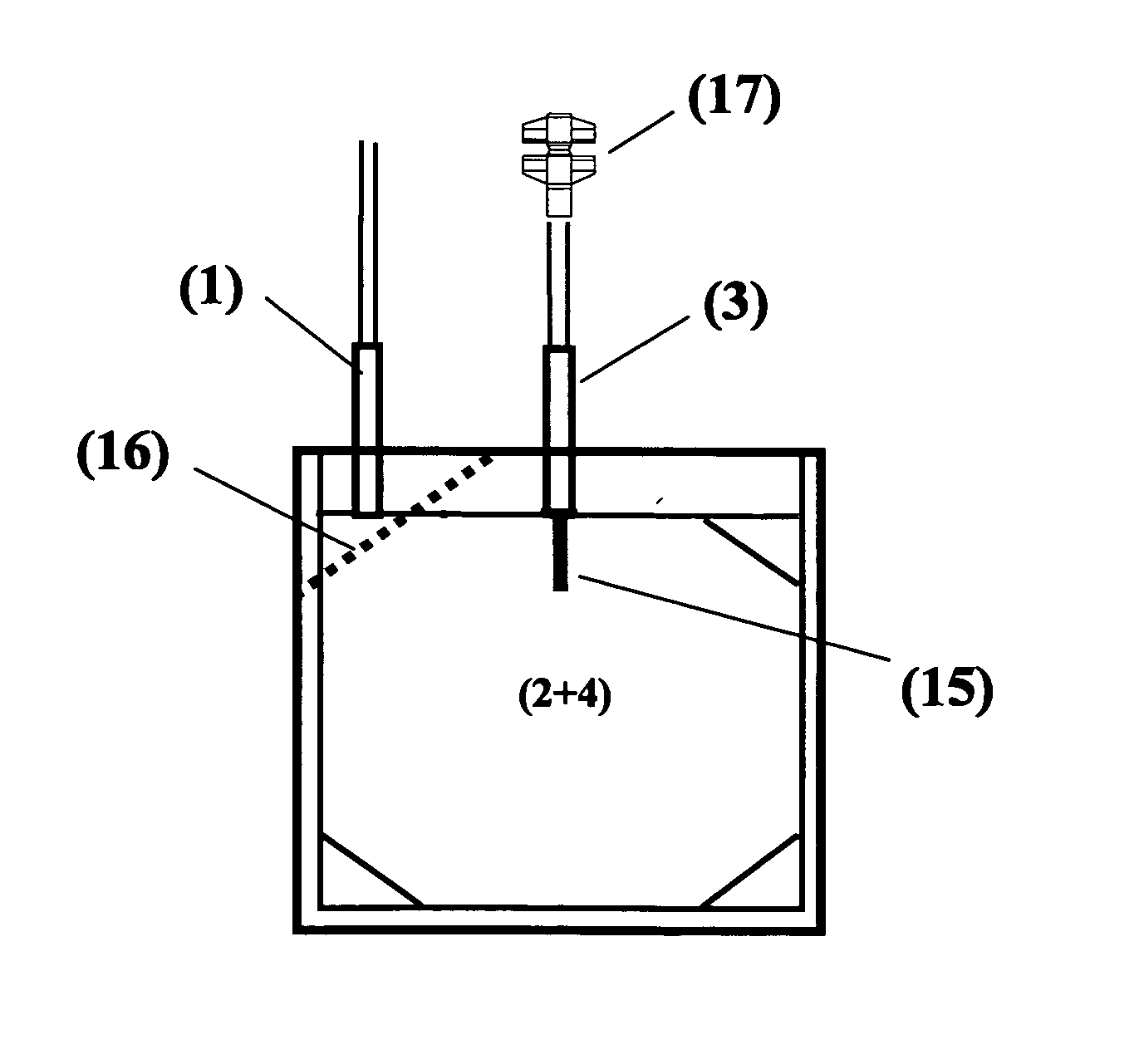
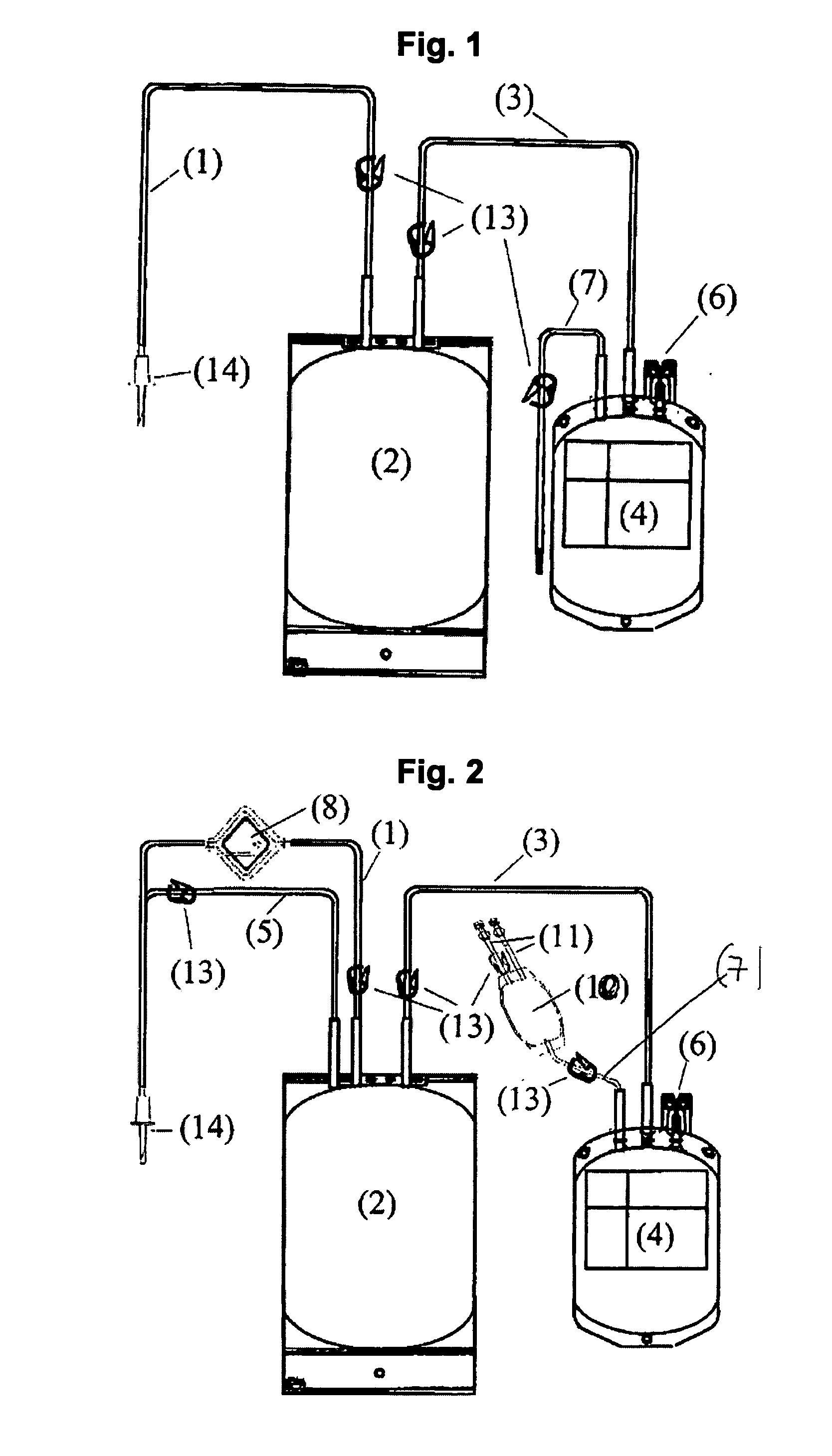
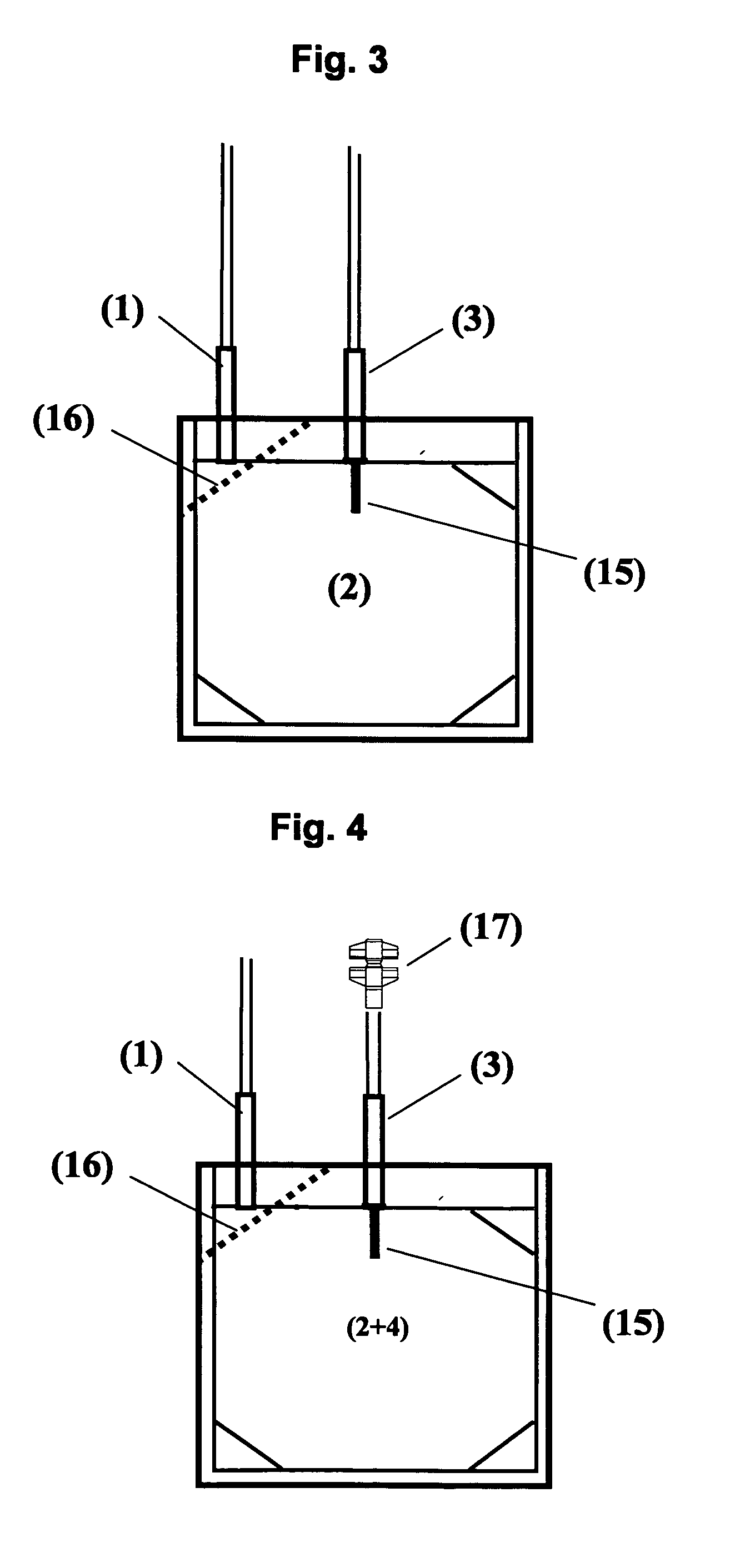
A therapeutic preparation (1) comprising ozonised oil and a platelet concentrate (2), mixed according to a mixing ratio between the volumes of the platelet concentrate (2) and of the ozonised oil (3) substantially in the range between 2 and 4.
Owner:安德烈亚·比尼奥蒂 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com