Blood bag system and process for the inactivation of pathogens in platelet concentrates by use of the blood bag system
a blood bag and platelet concentrate technology, applied in the field of blood bag system and process for inactivation of pathogens in platelet concentrates, can solve the problems of infectivity at the recipient, difficulty in ensuring the required degree of absence of pathogens in blood products, and the inability of the sengewald bag used in the method to avoid dead areas
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
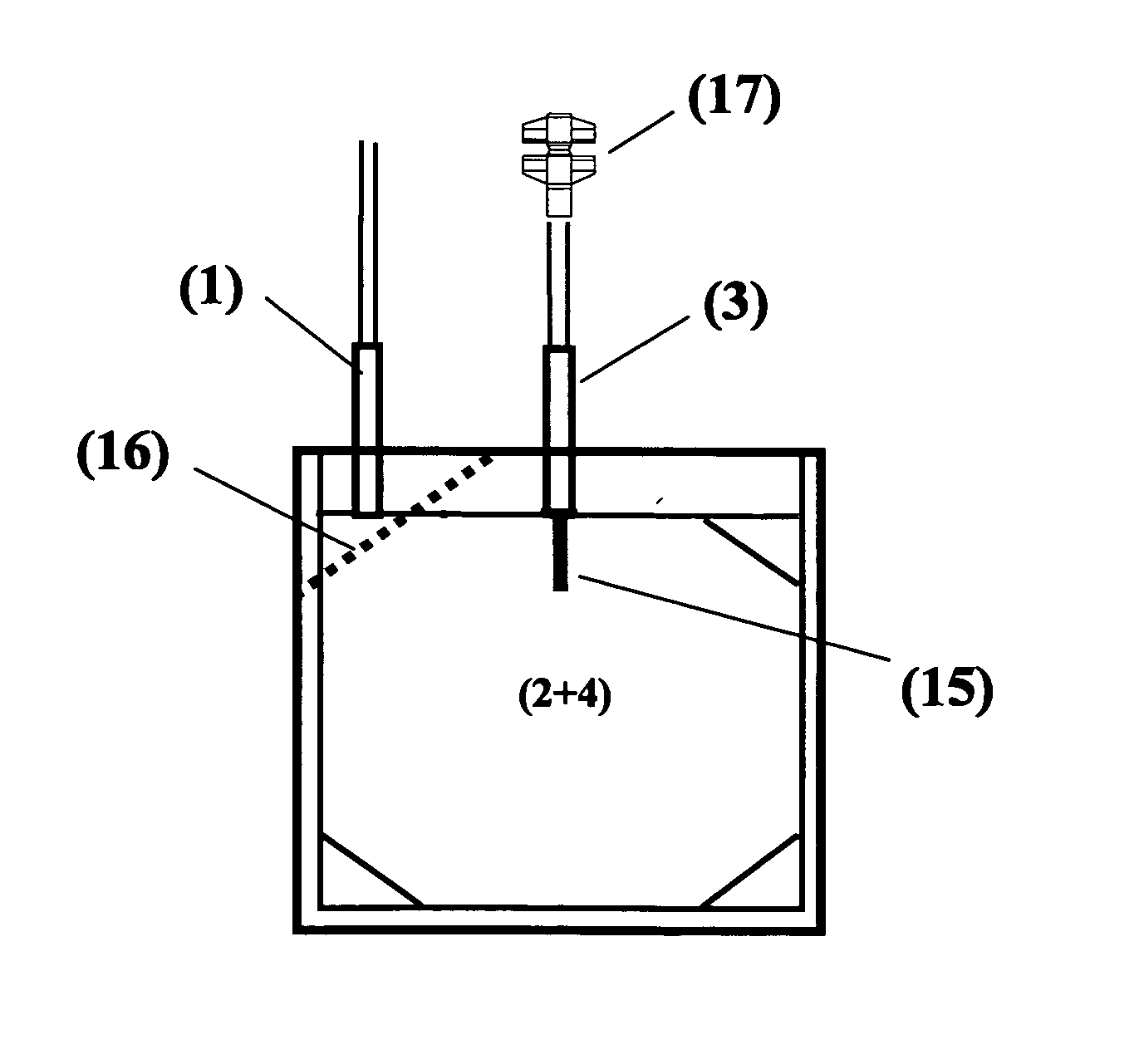
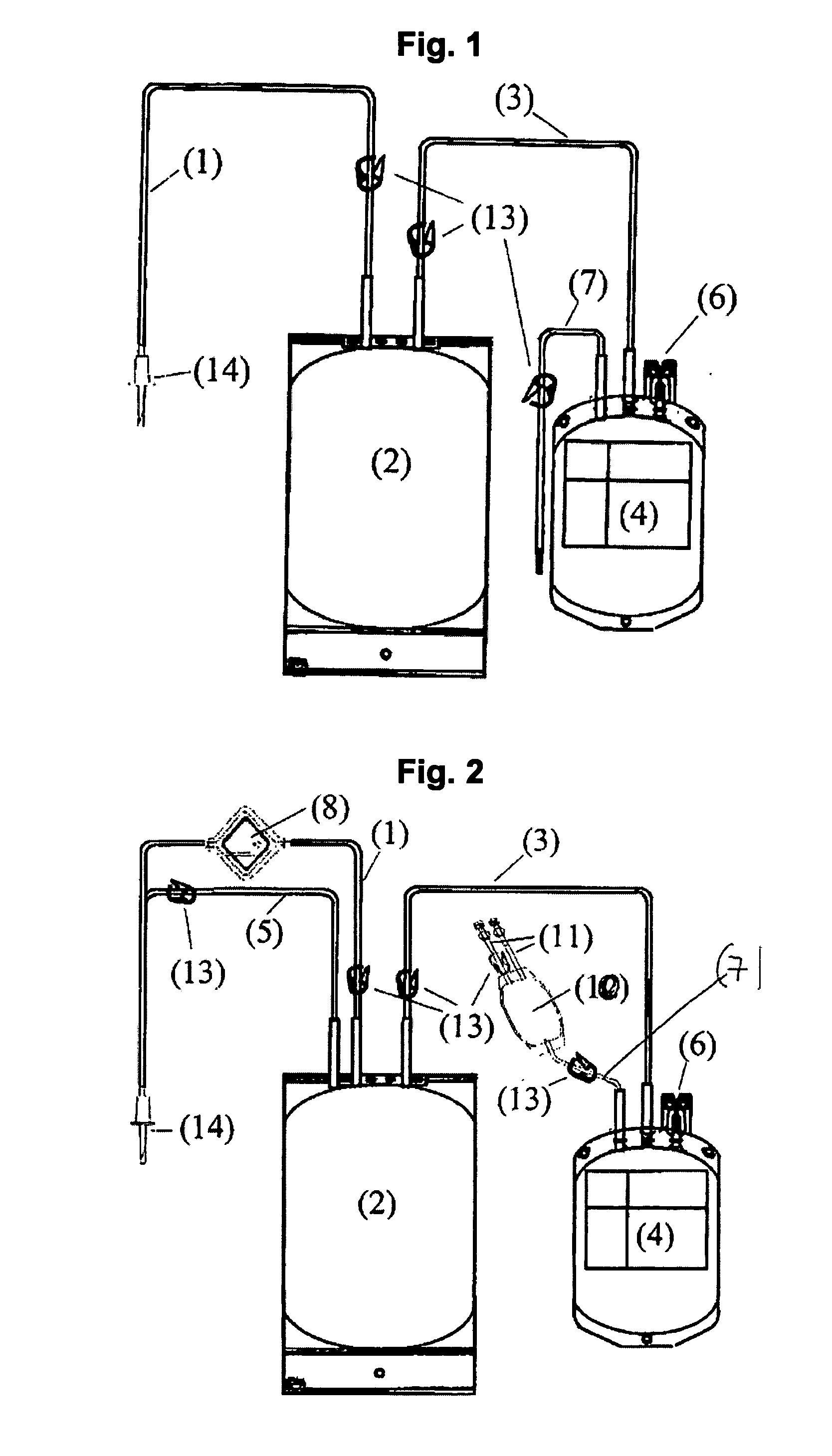
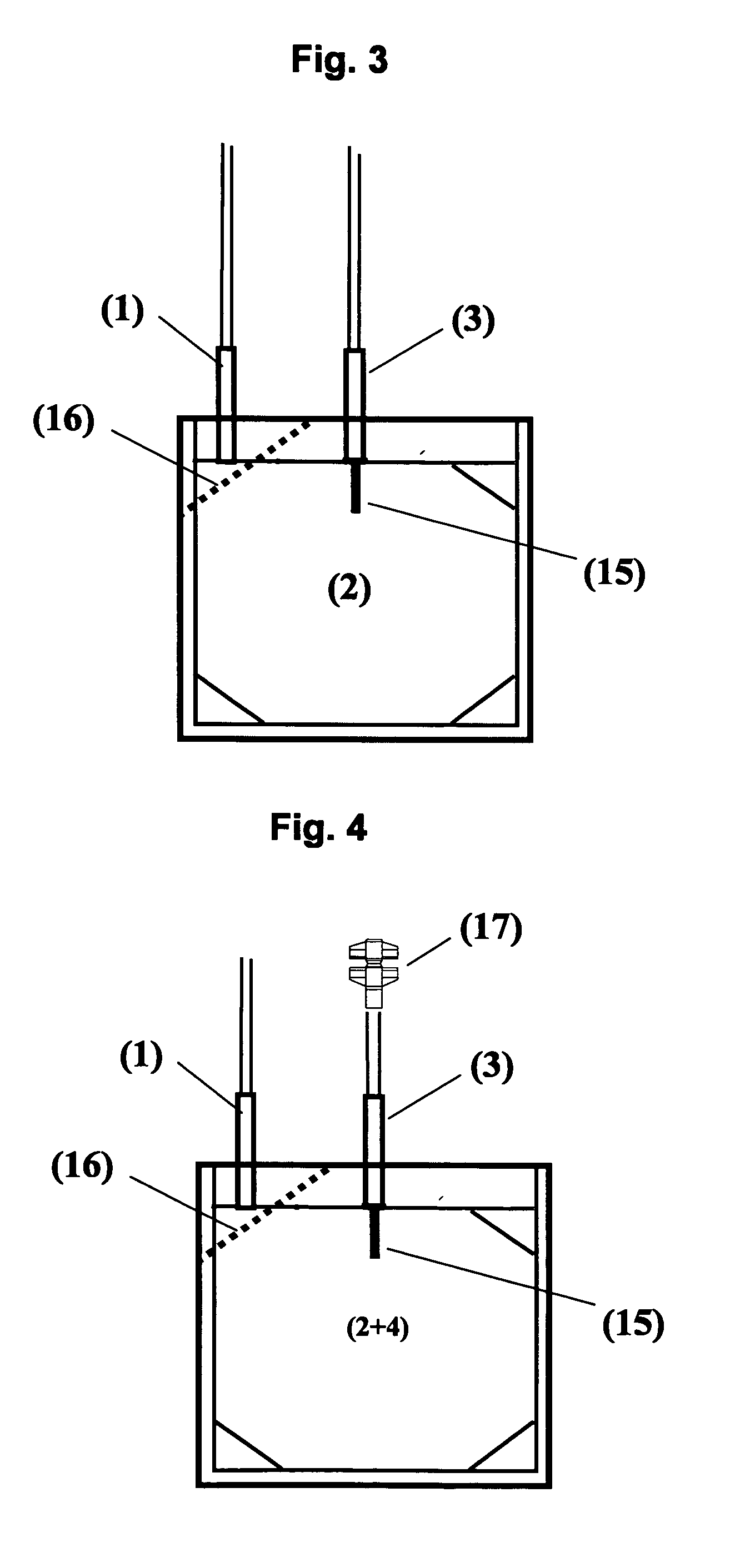
[0016]The blood bag system comprises either one bag for irradiation with UV light and storage of a suspended PC, wherein the irradiation bag forms at the same time the storage bag, or comprises a first bag for irradiation (irradiation bag) with UV light and a second bag (storage bag) for storage wherein in each of the different blood bag systems the irradiated suspended PC can be stored for up to 10 clays without clinically significant reduction of the therapeutic quality.
[0017]According to a preferred embodiment the blood bag system according to the invention comprises a leucodepletion filter for leucodepletion of the inlet stream of non-irradiated PC. The leucodepletion filter for above purpose is preferably incorporated in the inlet tubing of the irradiation bag.
[0018]The irradiation bag is made from an UV-transparent plastic material. Suitable polymer materials are polyolefins and ethylene vinyl acetate (EVA), extruded or calendered to wall thicknesses of 0.8 mm or less, in part...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com