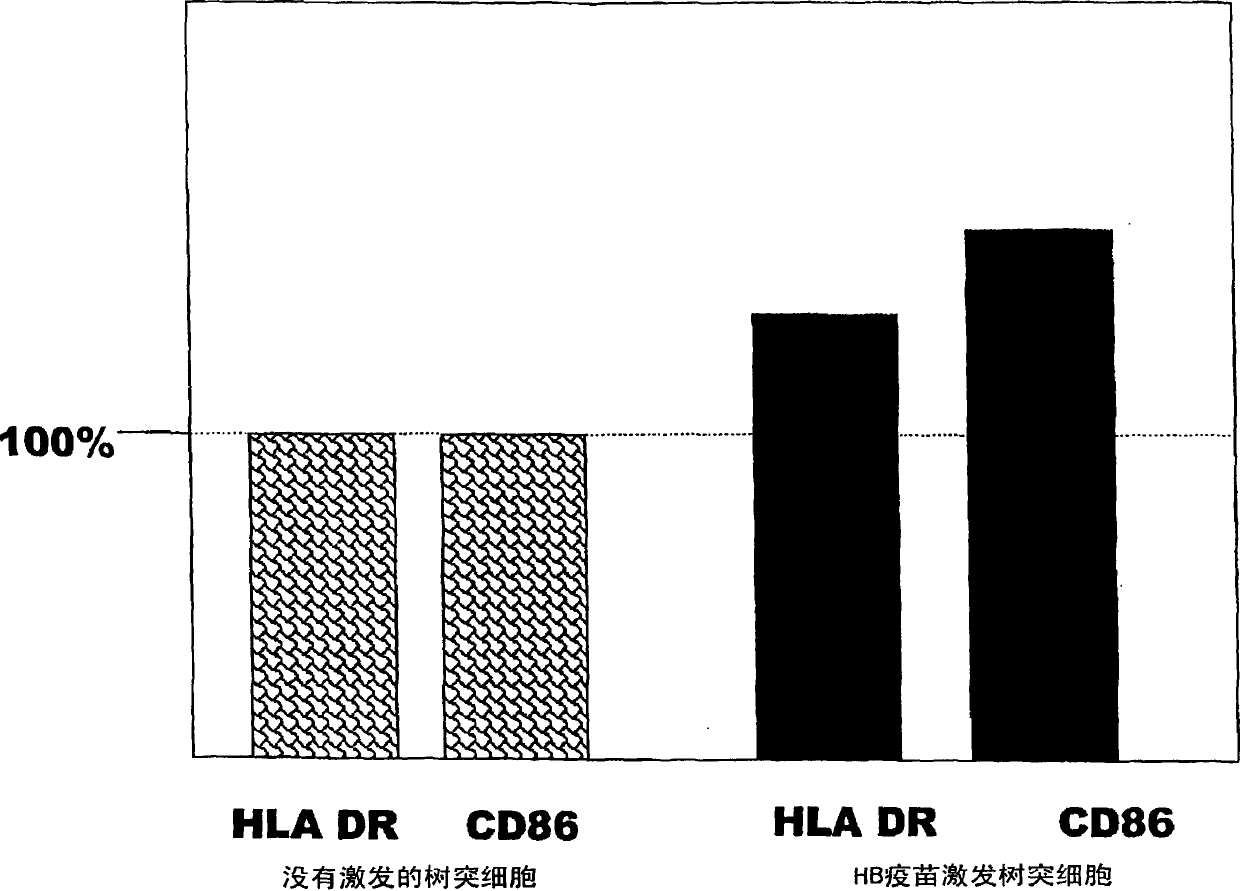
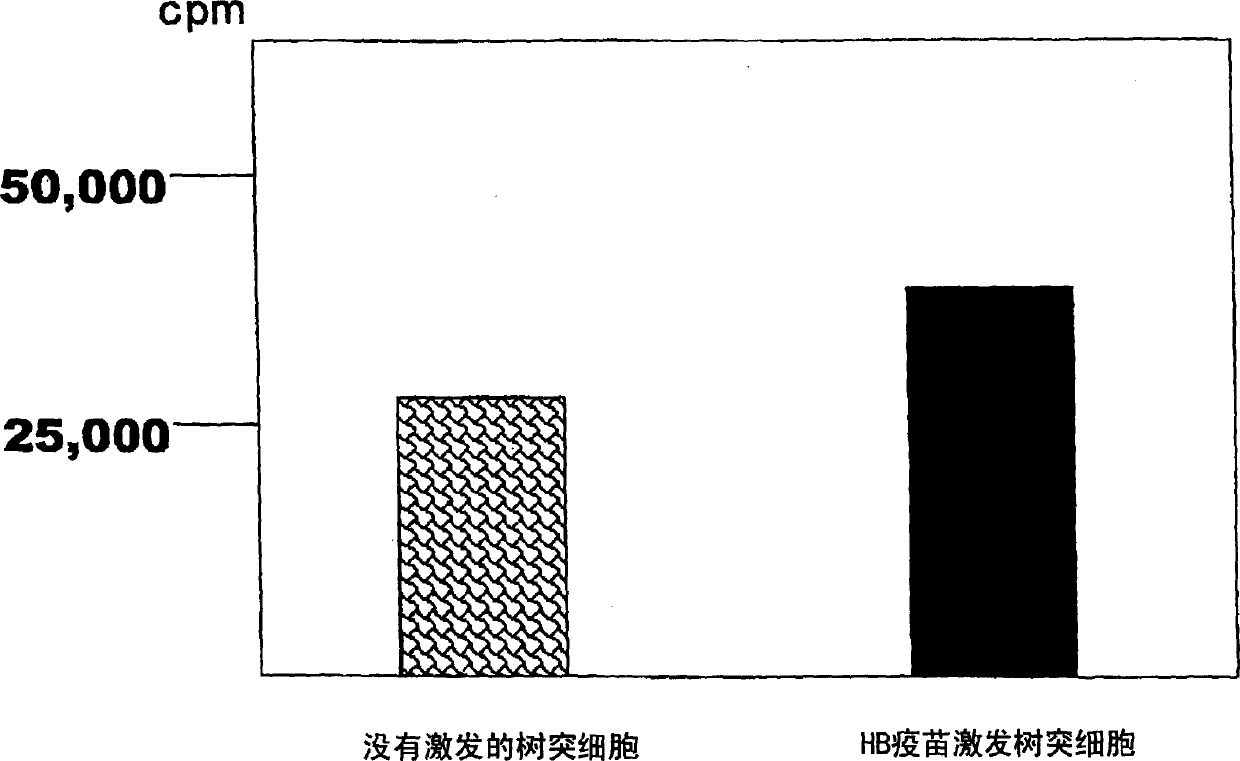
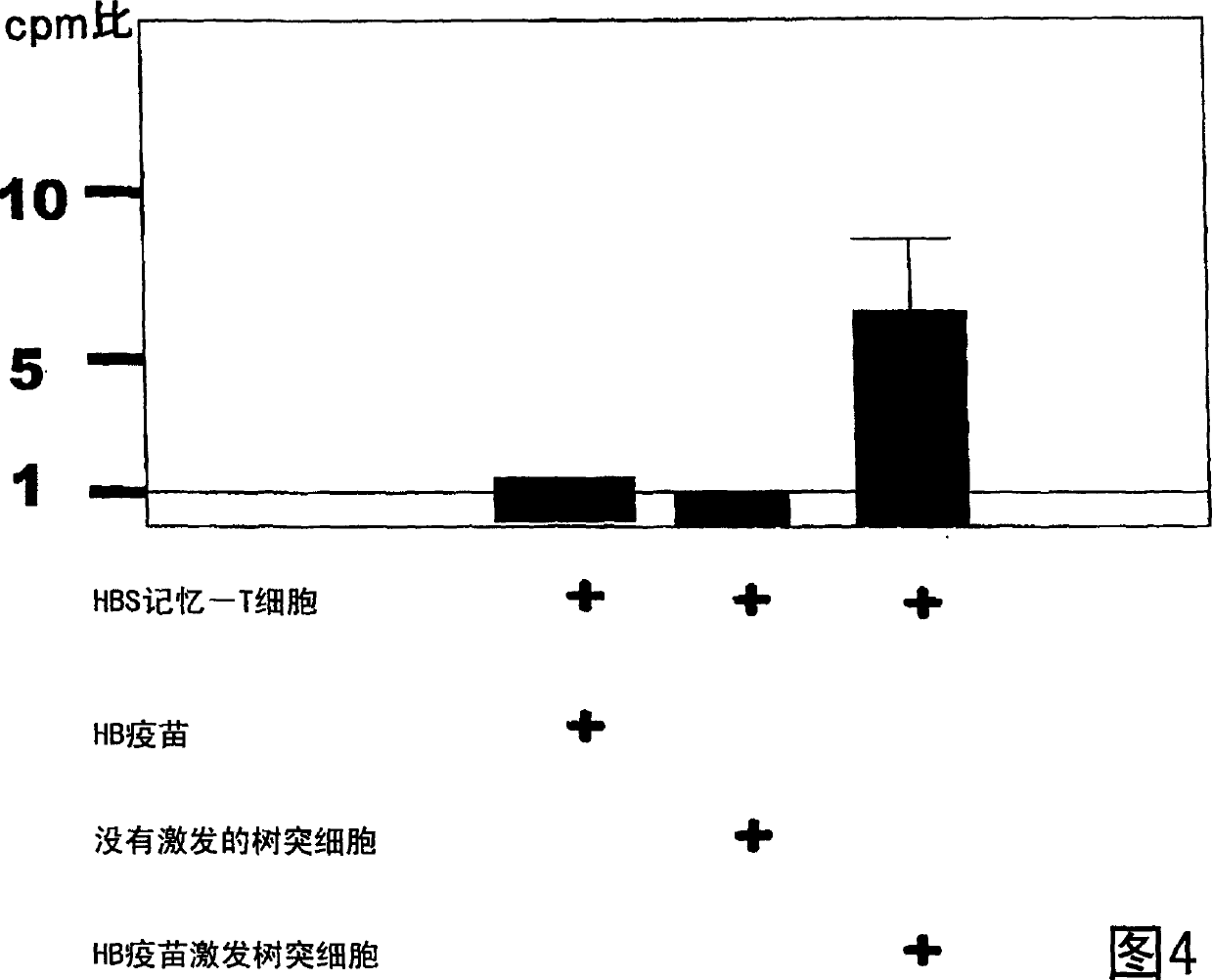
Dendritic cell obtained by antigen pulsing
A technology of dendritic cells and antigens, applied in animal cells, virus antigen components, vertebrate cells, etc., can solve the problems of impact on treatment performance, heavy economic burden, patients cannot produce anti-HBs antibodies, etc., and achieve the effect of improving immune response.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0118] The preparation method of dendritic cells with HBs antigen presenting ability related to this embodiment includes the step of stimulating dendritic cells with HBs antigen. Accordingly, dendritic cells capable of presenting HBs antigens that are effective in the prevention or treatment of hepatitis B can be obtained.
[0119] The above-mentioned preparation method may further include the step of obtaining the above-mentioned dendritic cells from blood. Accordingly, dendritic cells having HBs antigen-presenting ability according to the present invention can be obtained from blood-derived dendritic cells. In addition, if blood is used, dendritic cells can be obtained by a relatively simple operation using a conventionally known method or the like.
[0120] In addition, the above-mentioned preparation method may further include the step of collecting the above-mentioned blood from an organism.
[0121] The above-mentioned organisms may be humans. Accordingly, dendritic c...
Embodiment 1
[0200] The present invention will be specifically described below based on experimental examples, but the present invention is not limited to this example.
experiment example 1
[0201] (Experimental Example 1) Preparation of Dendritic Cells
[0202] (1) Using a syringe (Terumo Syringe SS-50ESZ: produced by Terumo Co.) moistened with heparin (Novo-heparin Note 1000: produced by Aventis Co.), from each subject (who has experience in repeatedly administering HB vaccine) 5 healthy volunteers) blood collection of 40-60ml. The obtained blood was fully mixed with the same amount of phosphate-buffered saline (PBS) as the blood in a special clean bench. Then, mononuclear cells were separated according to the specific gravity centrifugation method using Ficoll-Conray solution (specific gravity 1.077). Centrifuge at room temperature, 1800rpm-3000rpm for 30 minutes, and take out the middle layer after centrifugation as mononuclear cells.
[0203] (2) At the same time, autoserum was obtained from each subject according to the following steps. First, about 10 ml of blood was collected from each subject using another syringe (Terumo Syringe SS-10ESZ: manufactured...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com