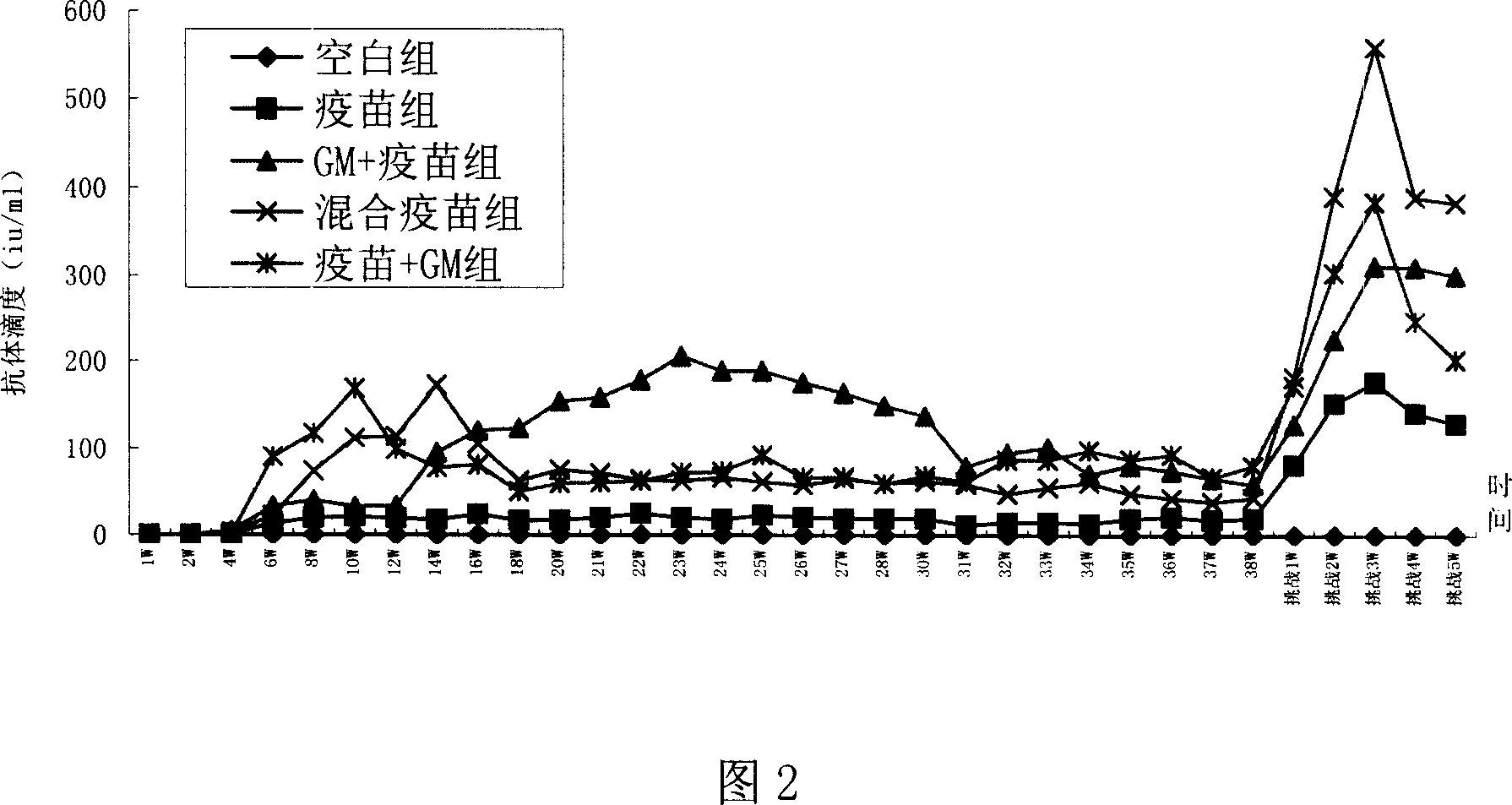
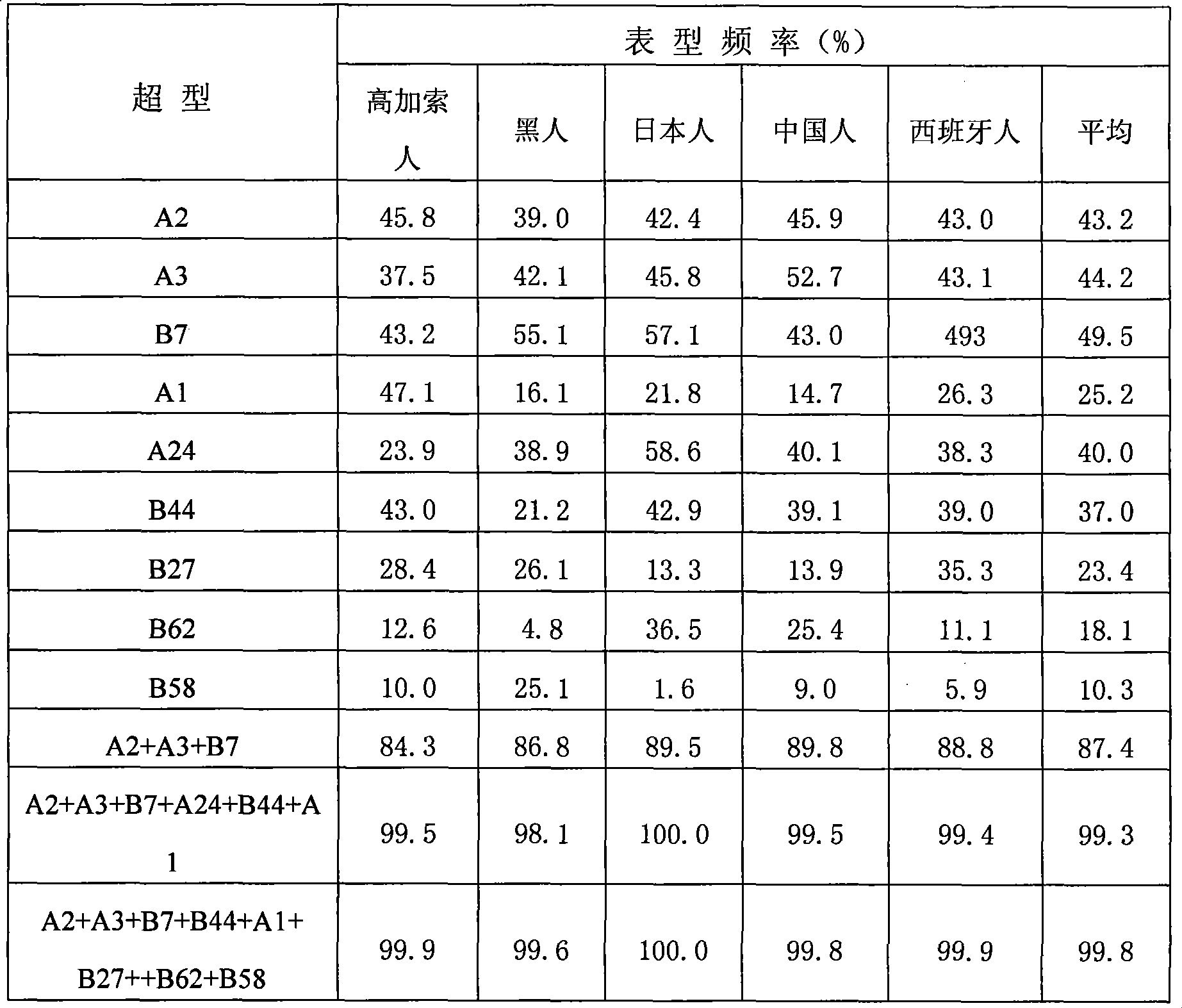
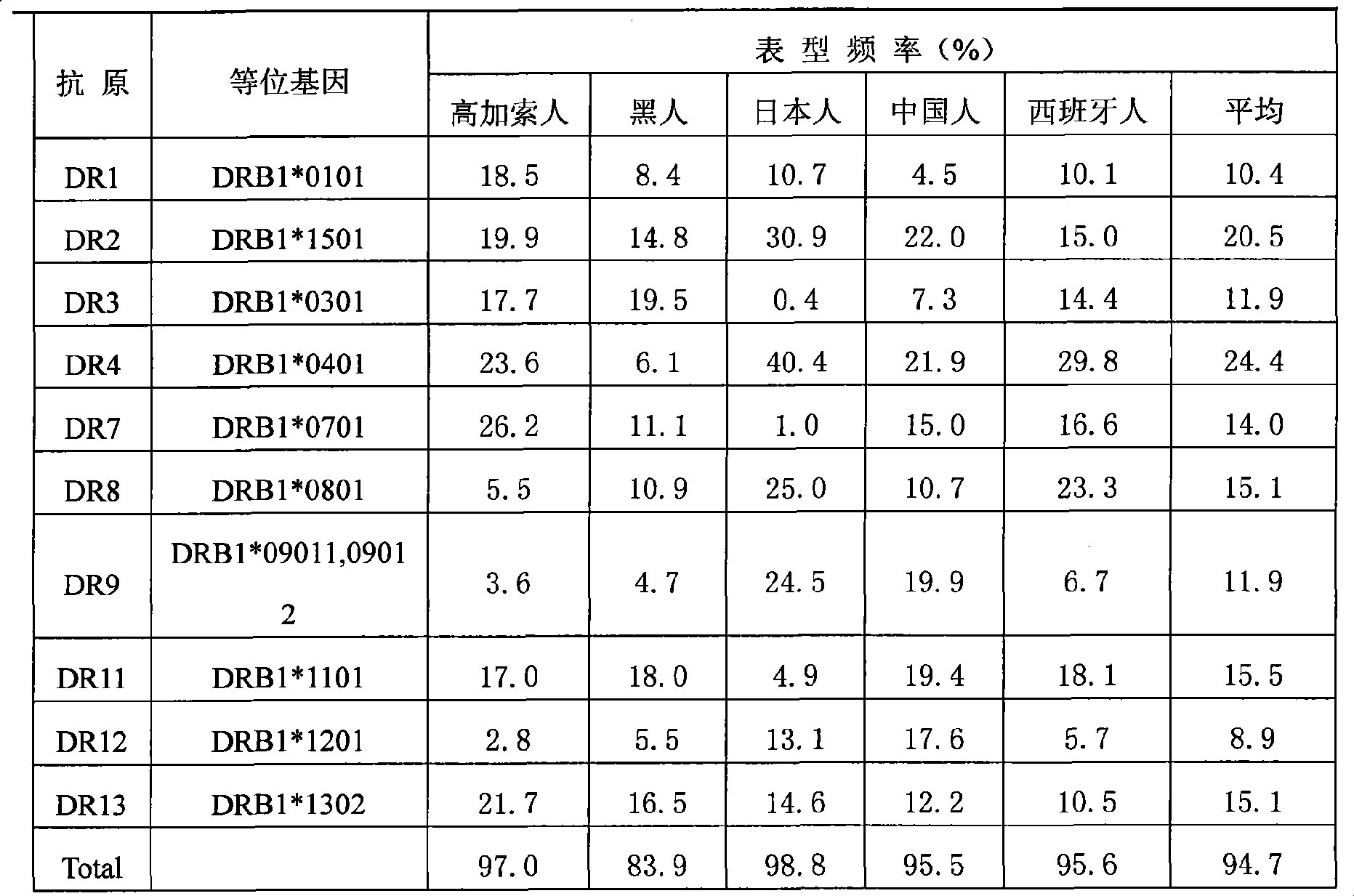
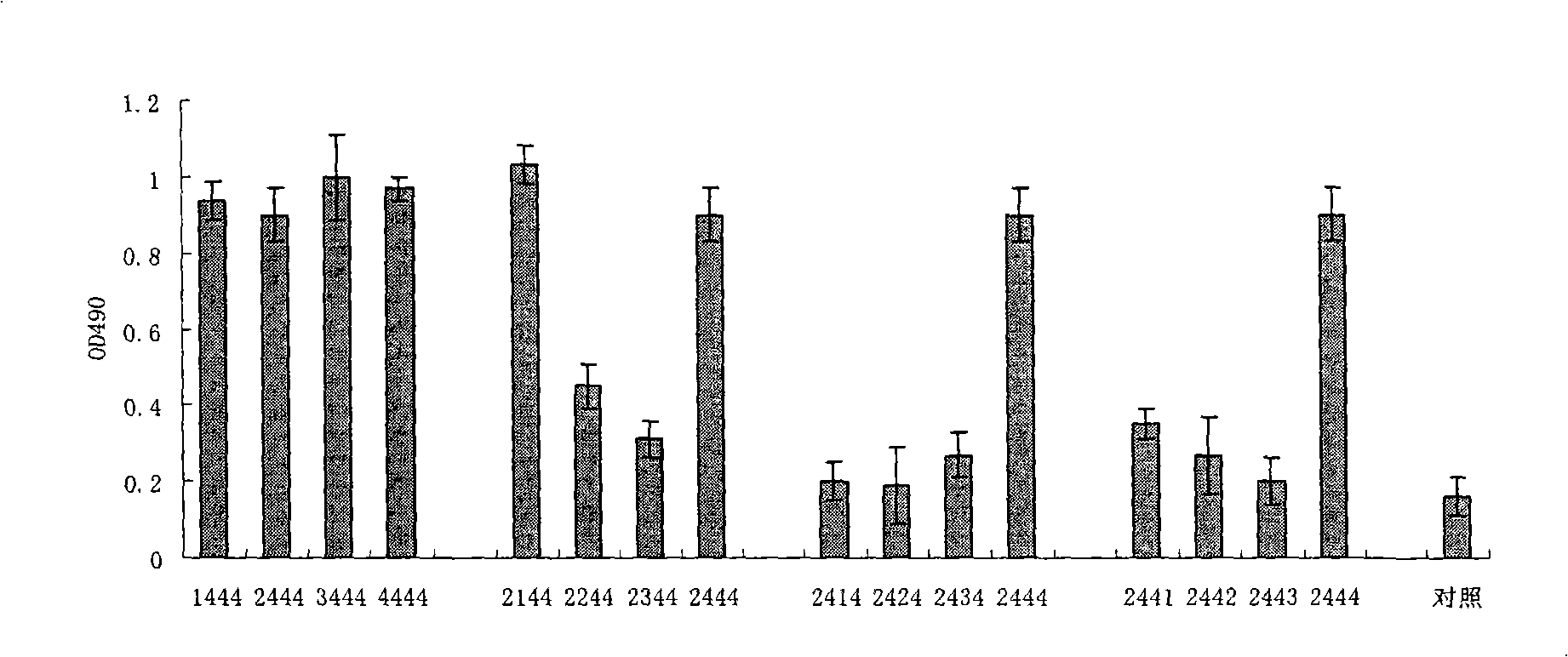Patents
Literature
128 results about "Hepatitis B virus vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hepatitis B vaccine is a vaccine that prevents hepatitis B. The first dose is recommended within 24 hours of birth with either two or three more doses given after that. This includes those with poor immune function such as from HIV/AIDS and those born premature.
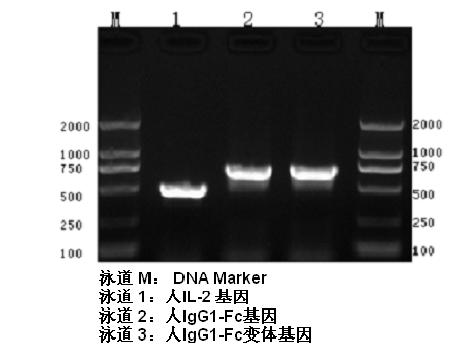
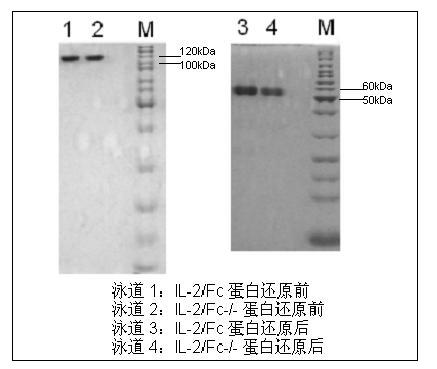
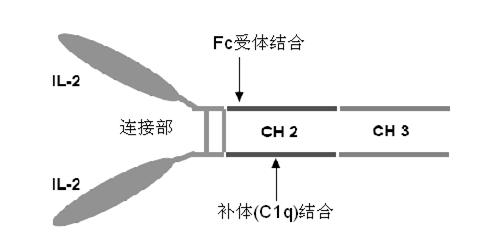
Human interleukin-2 (IL-2)/Fc fusion protein
ActiveCN102174111AEnhance humoral immune responseImprove immunityPeptide/protein ingredientsDigestive systemRegulatory T cellHalf-life
The invention provides human interleukin interleukin-2 (IL-2) / Fc fusion protein. The human IL-2 of the fusion protein comprises all sequences of a human IL-2 extracellular region; the Fc fragments comprise a hinge region, a CH2 region and a CH3 region; the human IL-2 / Fc sequences are fused directly or through a connection sequence; and the Fc fragments are human or animal IgG, IgM, IgD and IgA orsubtypes thereof. The ADCC and CDC effective factor action can be eliminated, and in addition, the human IL-2 / Fc fusion protein has the compatibility with a recombinant IL-2 receptor so that the half-life period is obviously prolonged and also has all the biological activity of the IL-2 receptor. The IL-2 / Fc obviously improves the humoral immune response stimulated by the hepatitis B vaccine and the immunity of the CD8+T cells targeted to the hepatitis B vaccine. Moreover, the balance immune (suppression) of the effective T cells and the regulatory t cells can be adjusted under the action of the cyclosporine A so that the pancreatic islet transplantation immune tolerance is induced.
Owner:上海百英生物科技股份有限公司
Vaccine for controlling persistent infection of hepatitis B virus
InactiveCN102038948AExcellent adjuvant effectPromote maturityAntiviralsAntibody medical ingredientsAdjuvantHepatitis B Surface Antigens
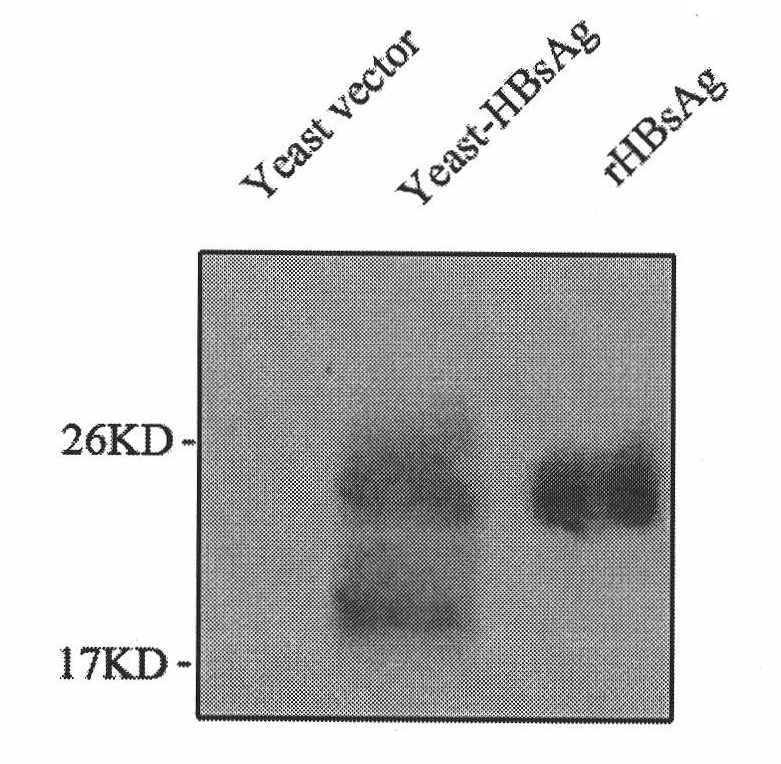
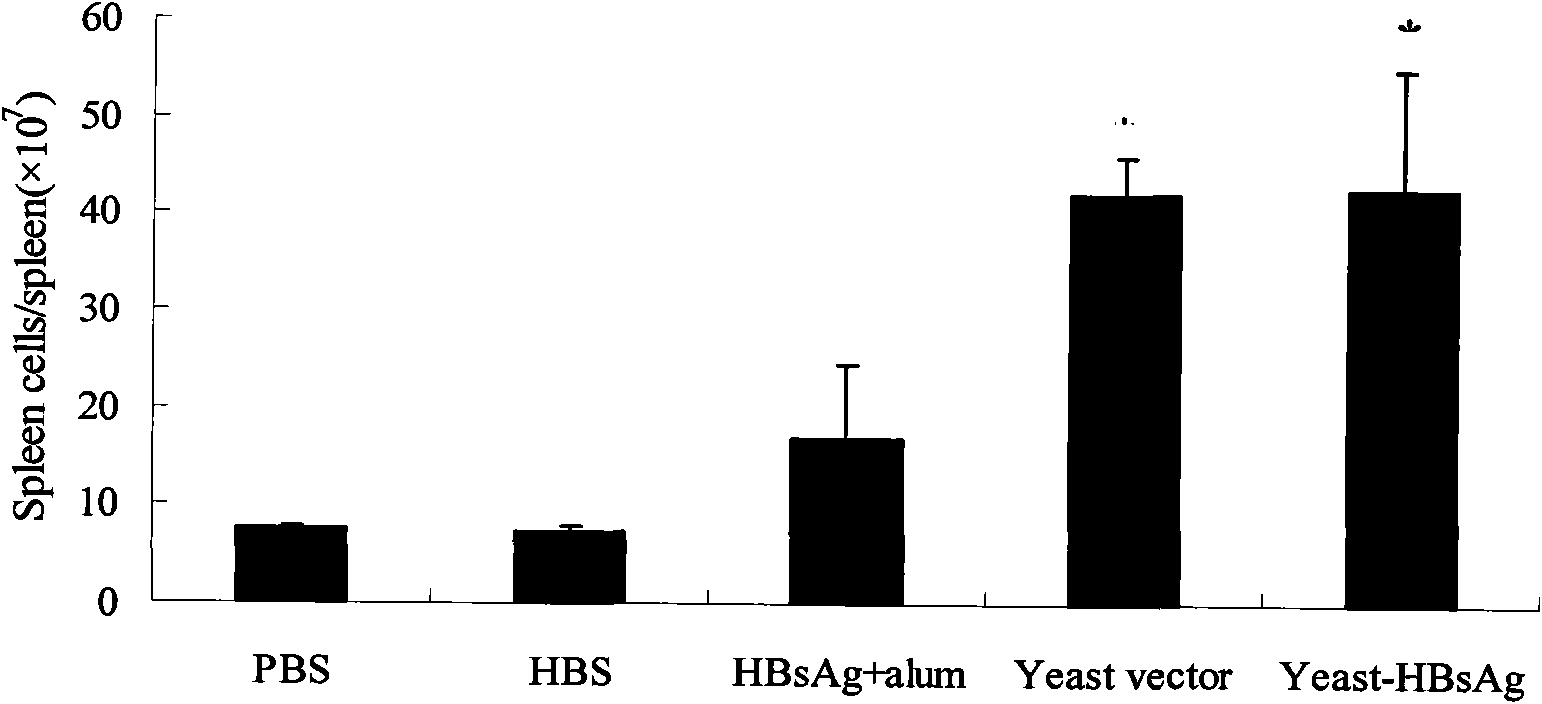
The invention belongs to the biotechnology field, and relates to a vaccine for controlling the persistent infection of hepatitis B virus. According to the invention, a Hansenula polymorpha cell that is heat inactivated and expresses hepatitis B surface antigen is adopted as the hepatitis B vaccine, wherein the HBsAg is an antigen part of the vaccine and the Hansenula polymorpha cell is an adjuvant part of the vaccine. Animal immunity experiment shows that: the vaccine can induce the aggregation of immune cells to the spleen, promote the maturation of DCs, and both induce Th1 immune response and enhance Th2 immune response in mice, including total IgG, IgG1, IgG2a, specific CTL activity, and the production ability of antigen-specific IFN-gama. The invention can make up for the deficiency of the impossibility of inducing Th1 immune response for traditional vaccines that take aluminium hydroxide as an adjuvant, and is helpful to the control of the persistent infection of hepatitis B virus.
Owner:FUDAN UNIV
Recombinant DNA sequence, hansenula polymorpha, preparation method for hepatitis B surface antigen, and hepatitis B vaccine
InactiveCN104232661AImprove expression levelSplit genetic stability is highFungiMicroorganism based processesChemical synthesisHigh cell
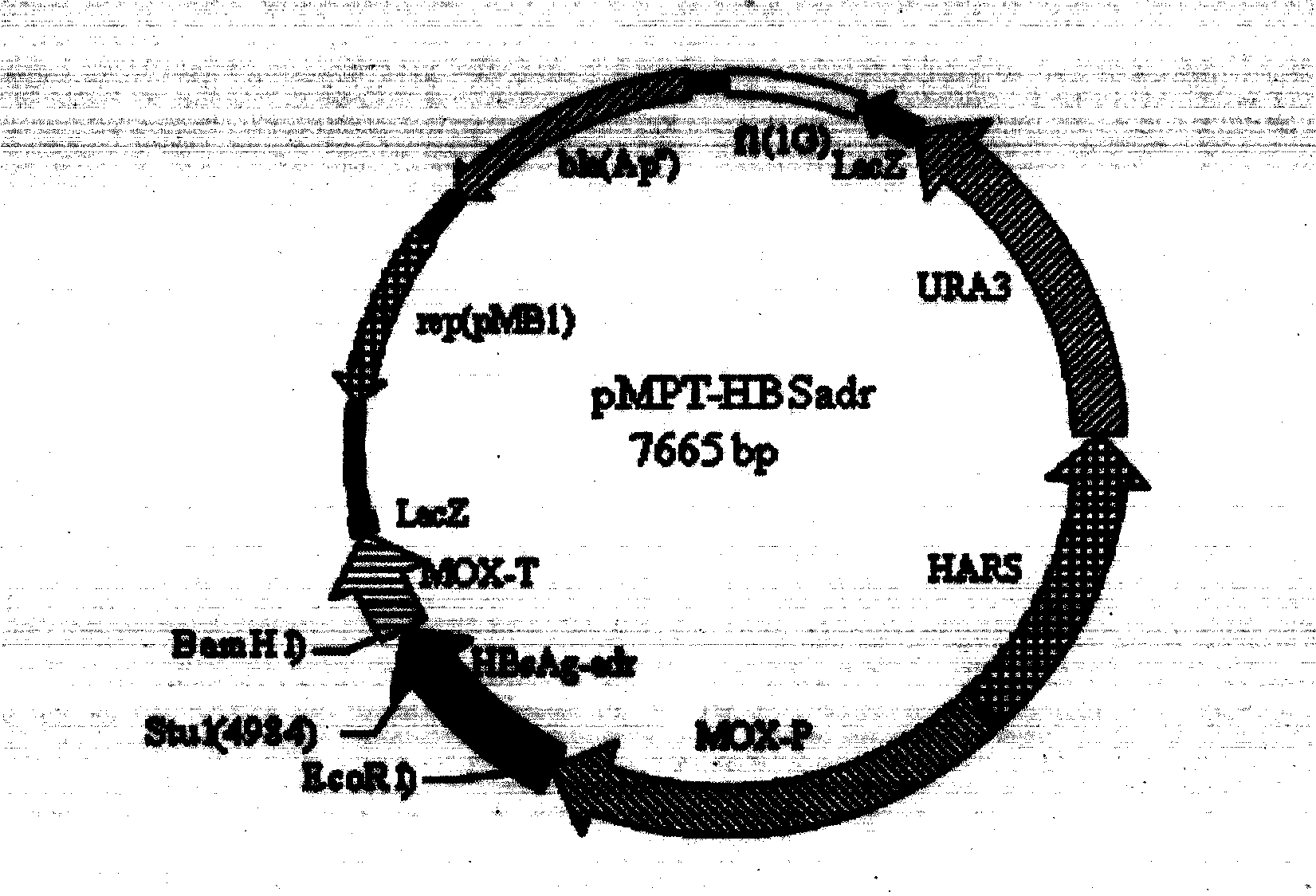
The invention provides a recombinant DNA sequence, hansenula polymorpha, a preparation method for hepatitis B surface antigen, and a hepatitis B vaccine. The recombinant DNA sequence is obtained by codon optimization of coding genes of the hepatitis B virus surface antigen according to codon usage frequency of the hansenula polymorpha. The invention also provides the hansenula polymorpha comprising the recombinant DNA sequence, a method for preparing adr sub-type hepatitis B surface antigen by using the recombinant DNA sequence, and the hepatitis B vaccine. The adr sub-type hepatitis B surface antigen has high expression level of the recombinant DNA sequence. The recombinant hansenula polymorpha is fast in growth speed, has high HBsAg yield, can be fermented in high cell density by using a cheap chemically synthetic medium, has low fermentation contamination rate and is beneficial to large-scale production; and HBsAg adr vaccine provided by the invention has high trend of Th1 and Th2 type cellullar immunologic response.
Owner:北京天坛生物制品股份有限公司
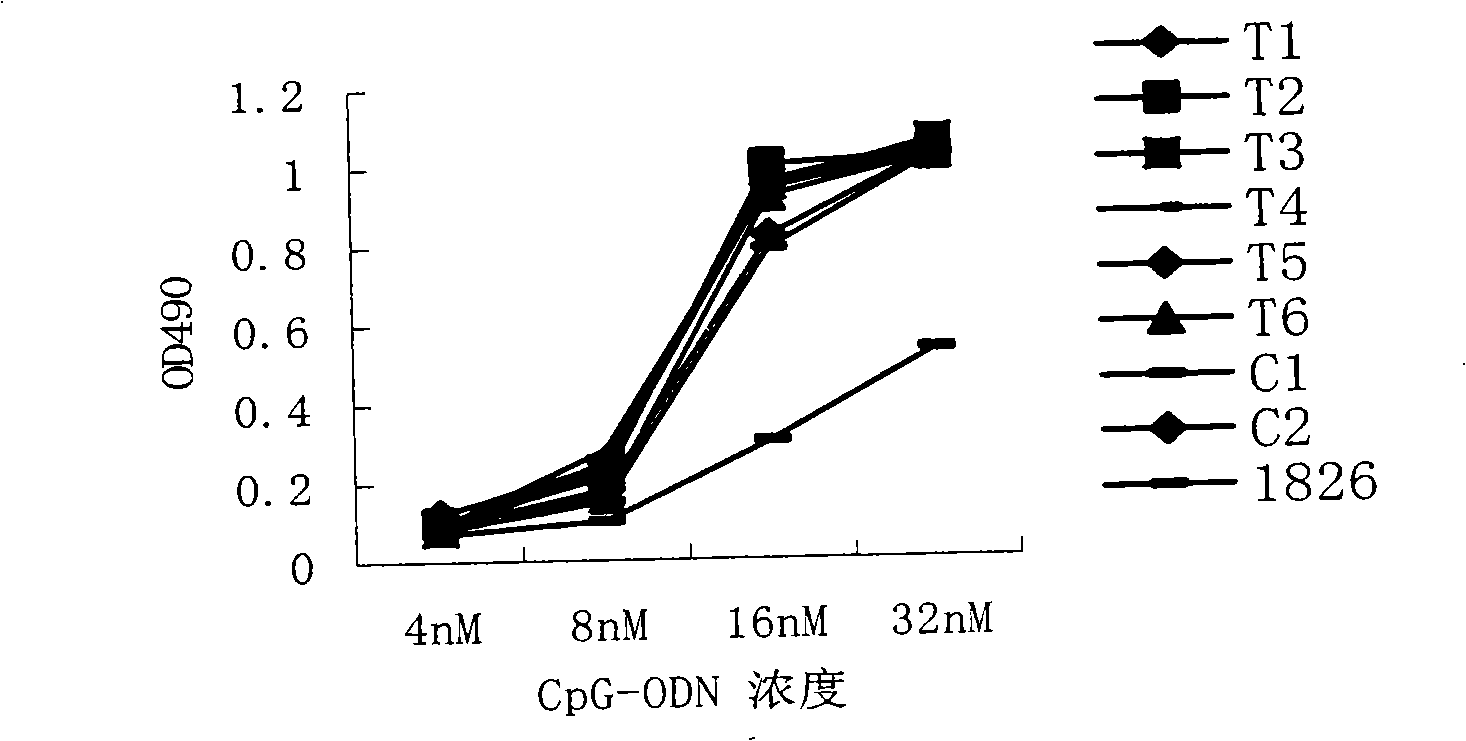
Sulpho-oligodeoxynucleotide with immune stimulation activity and uses thereof
ActiveCN101492672AHigh activityEnhance immune responseOrganic active ingredientsSugar derivativesNucleotideGene engineering
The invention relates to a phosphothioate oligodeoxynucleotide with immunostimulation activity. A 5'-NTCGTT-3' primitive with two or more than two copies is arranged in the sequence thereof and the length thereof is of 15 to 35 nucleotide; wherein, CpG is unmethylated; and N does not represent A or G. The phosphothioate oligodeoxynucleotide has excellent immunostimulation activity both to a human body and a mouse immunocyte in vitro; and the immunostimulation activity in the human body thereof can be estimated according to the results of immunostimulation activity to the mouse. As a vaccine adjuvant, the phosphothioate oligodeoxynucleotide can remarkably enhance the immune response of the mouse to the hepatitis B vaccine of gene engineering, and can lead the immune response to lean to the direction of TH1, thus can be used for controlling and curing hypersusceptibility and infection. Meanwhile, the phosphothioate oligodeoxynucleotide of the invention also has excellent activity to inhibit the growth of tumors in the body of the mouse.
Owner:许洪林
Therapeutic hepatitis B vaccine
ActiveCN102462840AInhibition of replicationDigestive systemAntiviralsActive componentHepatitis B Surface Antigens
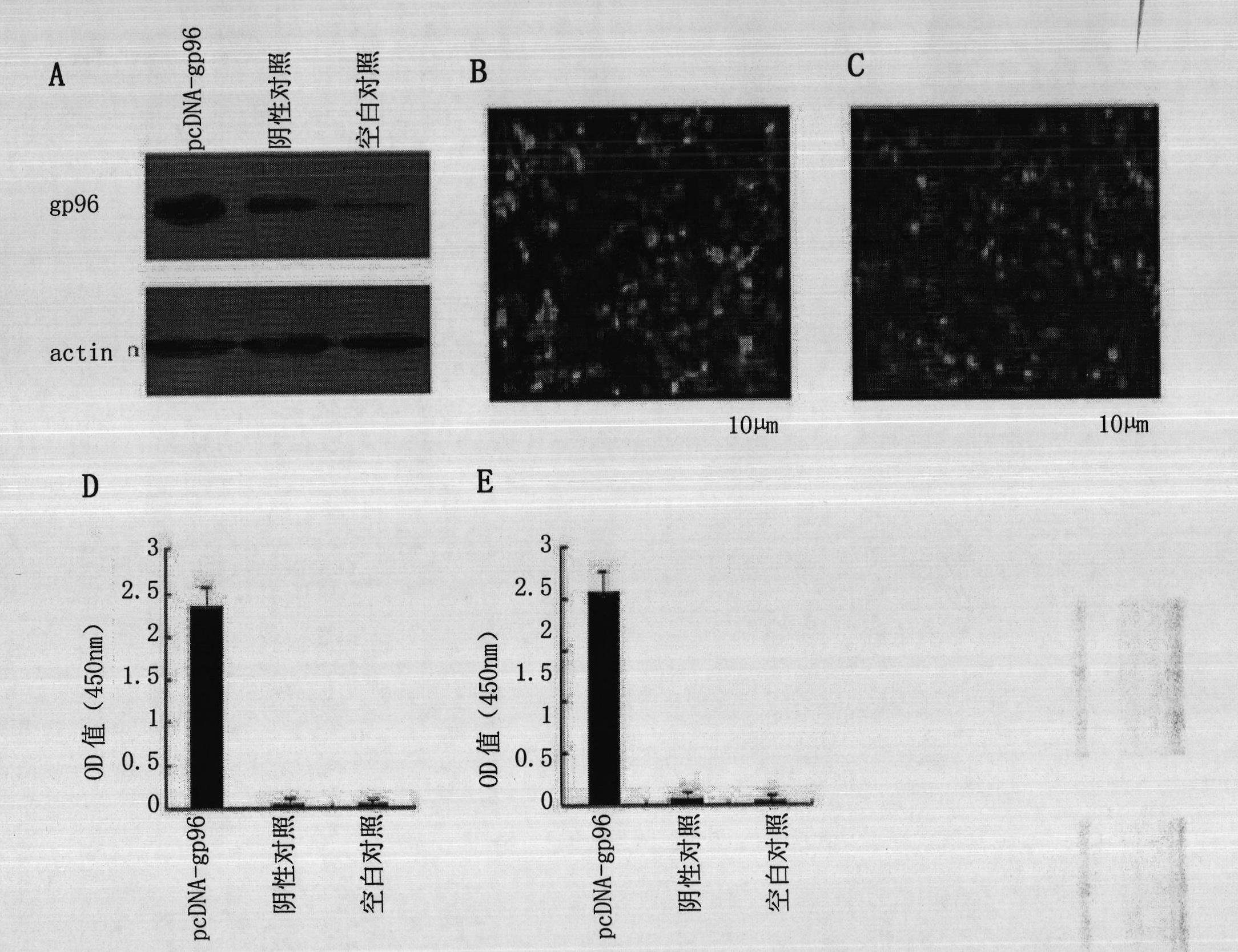
The invention discloses a therapeutic hepatitis B vaccine. Active components of the therapeutic hepatitis B vaccine comprise a protein gp96, a hepatitis B surface antigen and a hepatitis B core protein. The protein gp96 has a sequence shown in the sequence 1 of the sequence table. The hepatitis B surface antigen has a sequence shown in the sequence 5 of the sequence table. The hepatitis B core protein has a sequence shown in the sequence 3 of the sequence table. The active components also comprise a plasmid pcDNA-gp96 containing a coding gene of the protein gp96, a plasmid pcDNA-HB containing a coding gene of the hepatitis B surface antigen and a plasmid pcDNA-HBc containing a coding gene of the hepatitis B core protein. The therapeutic hepatitis B vaccine provided by the invention can effectively inhibit hepatitis B virus (HBV) replication, can eliminate viruses infecting the liver and has a very important value to hepatitis B prevention and treatment.
Owner:北京热休生物技术有限公司
Use of recombinant human granulocyte macrophagocyte colony stimulating factor in preparation of drug for preventing and treating hepatitis B
ActiveCN1990043APeptide/protein ingredientsDigestive systemColony-stimulating factorGenetic engineering
The invention discloses a use of recombinant human granulocyte macrophage colony stimulating factor in the preparation of treatment or prevention hepatitis B, while includes simultaneous or successive given recombinant human granulocyte macrophage colony stimulating factor and genetic engineering hepatitis B vaccine for the prevention or treatment of hepatitis B.
Owner:华北制药金坦生物技术股份有限公司
Therapeutic hepatitis b vaccine and preparation method and use thereof
ActiveCN101361969AOvercome the defect of low antigenicityStrong specificityPeptide/protein ingredientsAntiviralsAdjuvantWhite blood cell
The invention discloses a curative hepatitis B vaccine and a preparation method and applications thereof. The key of the technical proposal is that: a plurality of CTL and HTL polypeptide epitopes originating from HBV envelope protein, core antigen and polymerase are selected to form a multiple antigenic peptide (MAP) system and recombinated human interleukin 12 (rhIL-12) is adopted as an adjuvant, so as to improve the immunogenicity and the response level, thus providing a biological preparation for curing chronic hepatitis B clinically.
Owner:广州市茵良强生物科技有限公司
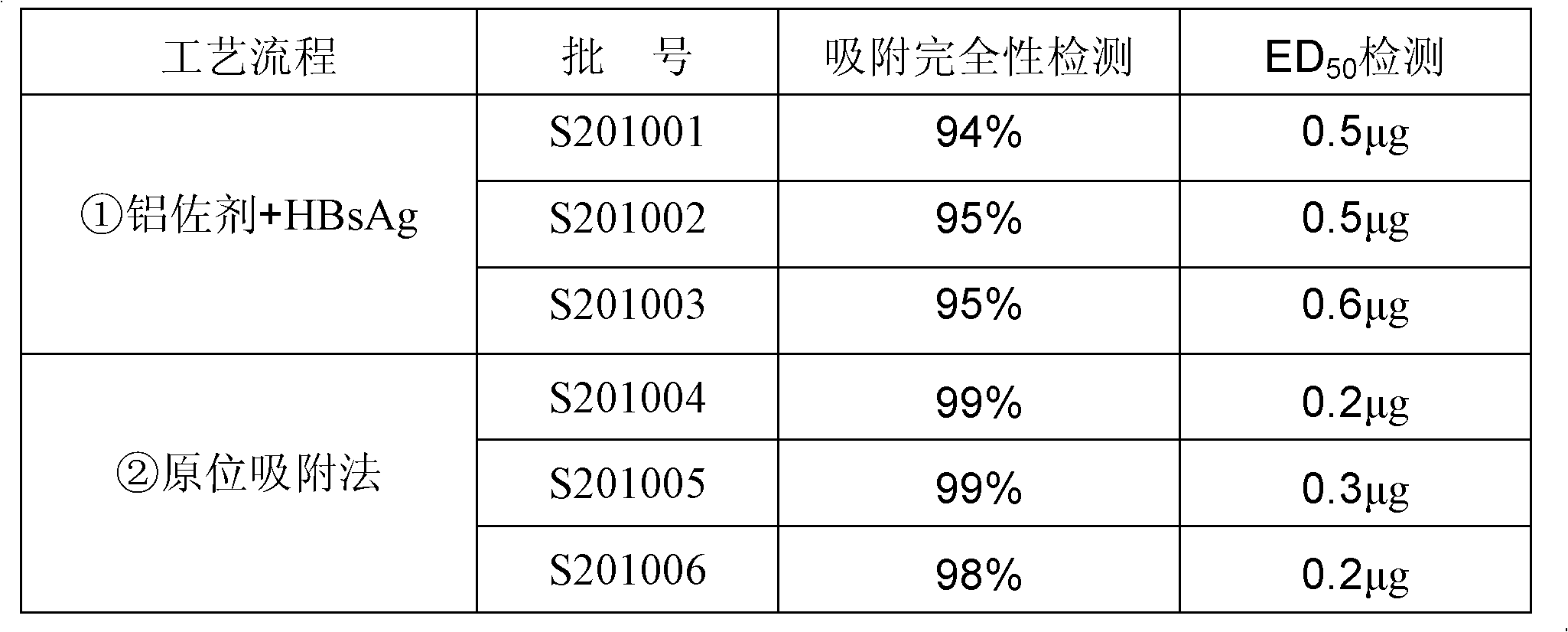
Preparation method of aluminum-containing adjuvant hepatitis B vaccine
ActiveCN102198270AIncrease inoculum volumeHigh positive conversion rateViral antigen ingredientsDigestive systemAdjuvantIn situ adsorption
The invention discloses a preparation method of an aluminum-containing adjuvant hepatitis B vaccine, belonging to the biotechnology field. The preparation method is characterized in that an aluminum adjuvant Al(OH)3 is produced by an on-line reaction, i.e. after a phosphate buffer solution (PBS), a KAl(SO4)2 solution and a hepatitis B surface antigen stock solution are mixed, an NaOH solution is added to the mixed solution, an Al(OH)3 adjuvant is continuously produced, and simultaneously, hepatitis B surface antigens are continuously coated and adsorbed; and the process is called 'in-situ adsorption'. In the invention, the Al(OH)3 adjuvant is produced by an in-situ reaction to greatly improve the adsorption rate of the hepatitis B surface antigens, thereby improving the immunogenicity of the antigens, being capable of more effectively causing organisms to generate an immune response, and producing more protective antibodies. The practice proves that the aluminum adjuvant hepatitis B vaccine produced by the method disclosed by the invention has the advantages of small inoculation amount, few adverse responses, high antibody positive conversion rate and the like, and can induce high-level antibody response after being immunized. Simultaneously, the processing steps are also simplified, and the production cost is greatly lowered.
Owner:DALIAN HISSEN BIO-PHARM CO LTD
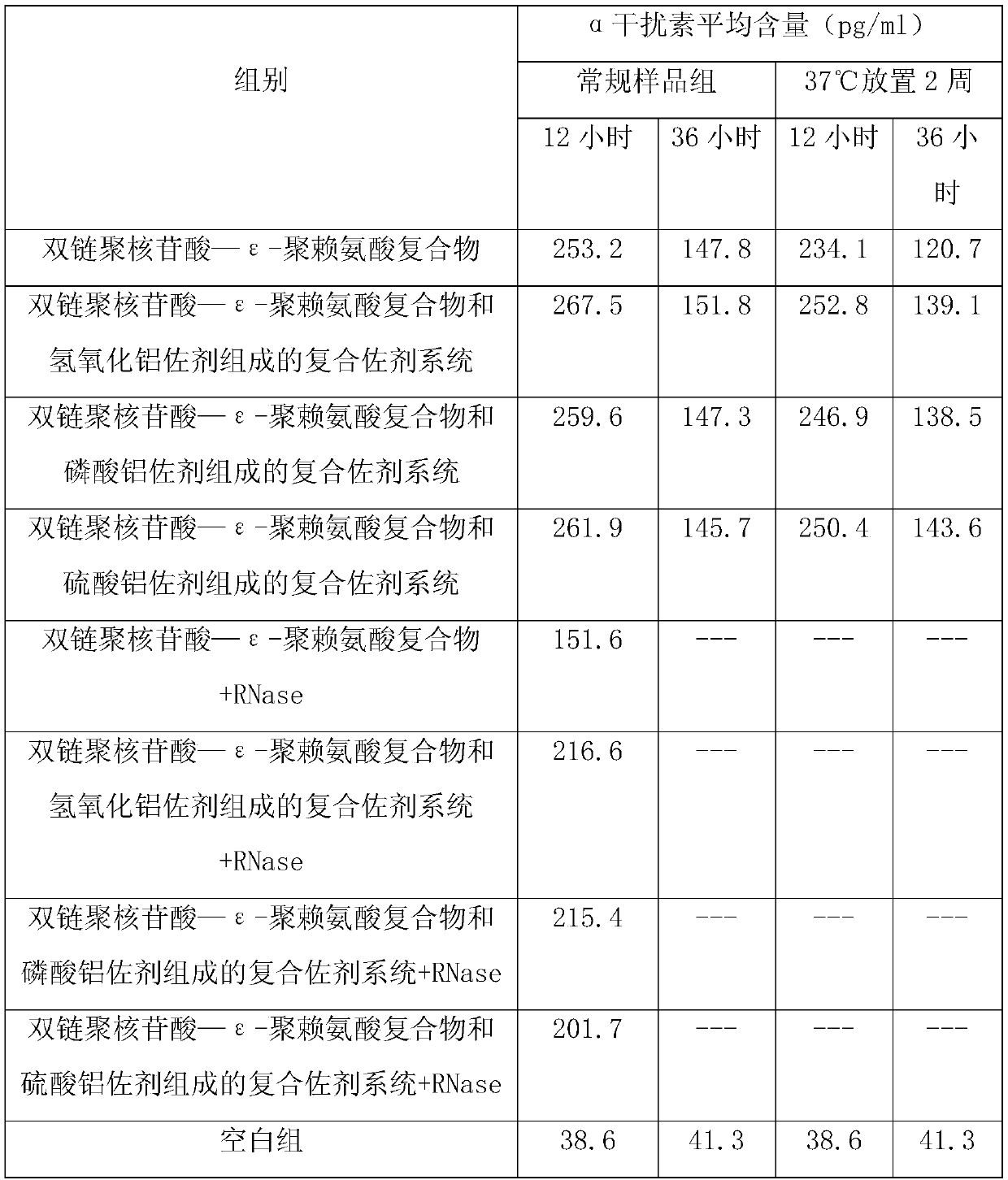
Vaccine composite adjuvant system and application thereof in antigens
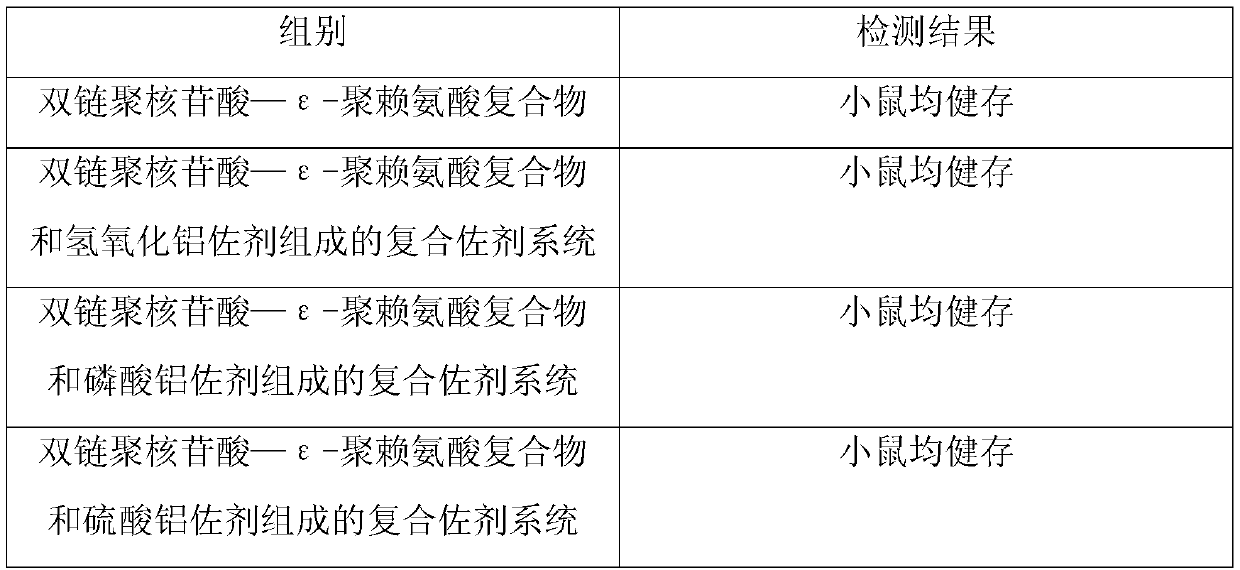
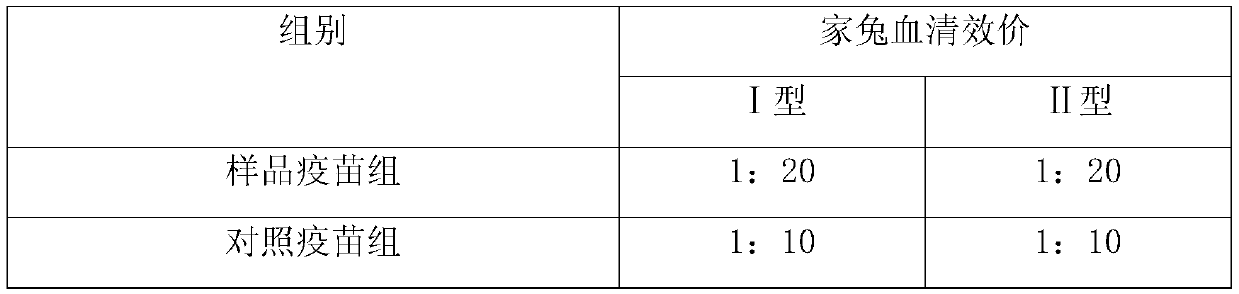
ActiveCN109701010AStrong hydrolysis abilityImprove stabilityAntiviralsImmunological disordersEpsilon-PolylysineAdjuvant
The present invention provides a vaccine composite adjuvant system and an application thereof in antigens. The vaccine composite adjuvant system is composed of an aluminum adjuvant, a double-strandedpolynucleotide-epsilon-polylysine complex and an aqueous solvent, wherein a clinical use concentration of the aluminum adjuvant is 0.1-10.0 mg / ml, and a clinical use concentration of the epsilon-polylysine complex based on double-stranded polynucleotide is 100-10,000 [mu]g / ml. The constructed compound adjuvant system has good anti-RNase hydrolysis ability, stability, safety and immune stimulatingactivity, is also combined with different forms of vaccines of hepatitis B vaccine, inactivated rabies vaccine, etc., and can also significantly improve immunogenicity of the vaccines.
Owner:辽科生物(沈阳)有限公司
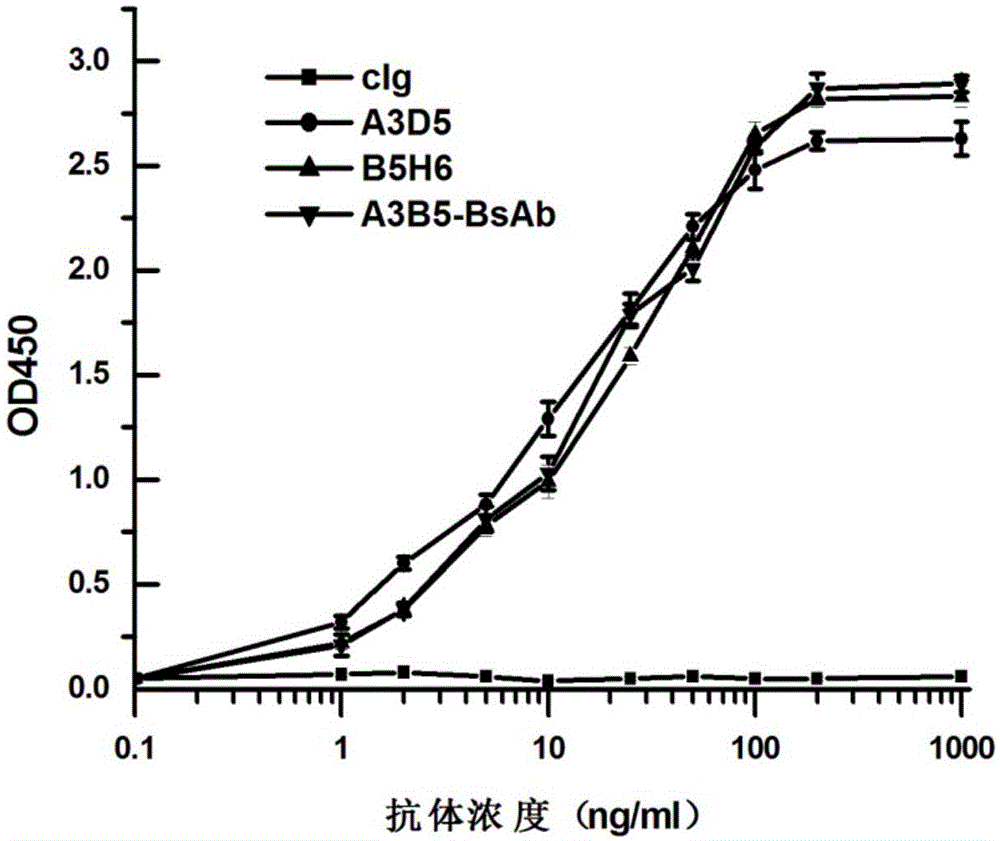
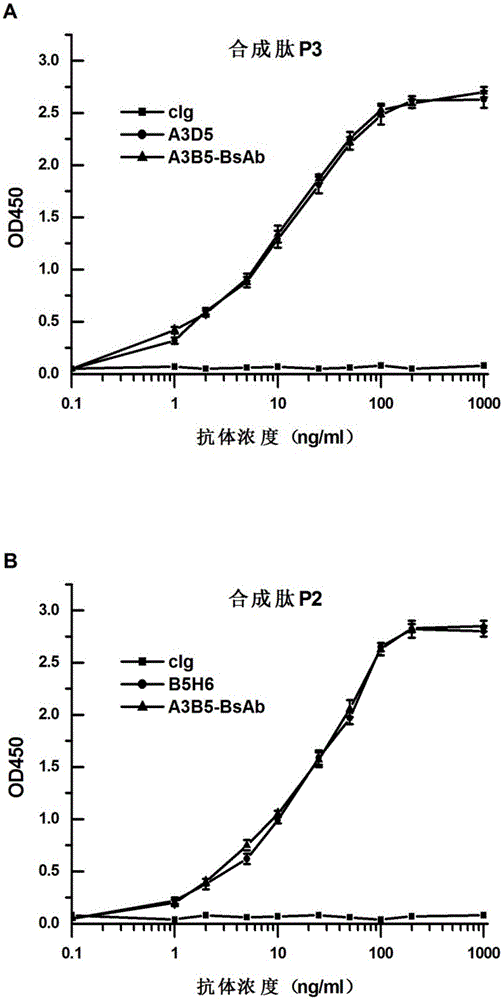
Bispecific antibody for hepatitis B surface protein, and use thereof
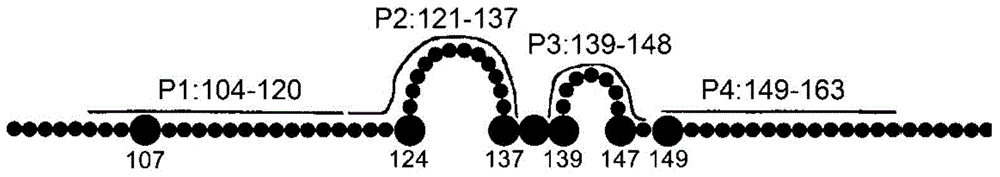
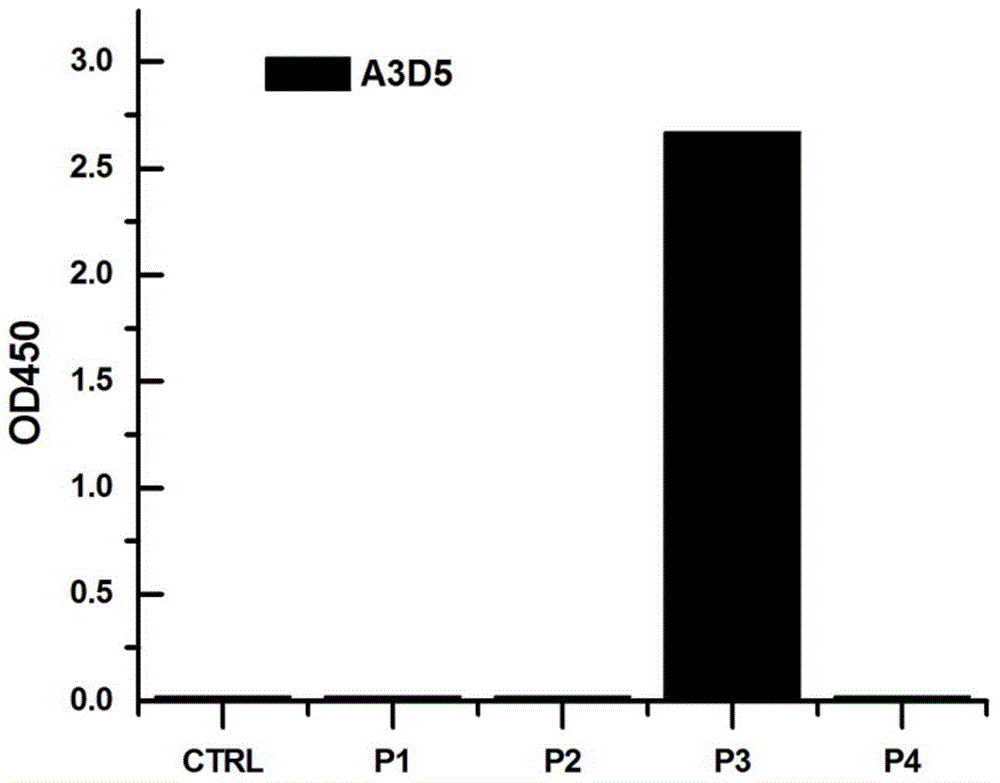
ActiveCN105061590AAvoid infectionCombined Application ExcellenceImmunoglobulins against virusesAntiviralsHepatitis a+b vaccineHumanized antibody
The invention provides a bispecific antibody A3B5-BsAb for a hepatitis B surface protein. The bispecific antibody is formed by combining an antibody A3D5 with an antibody B5H6. The bispecific antibody has better HBV virus neutralization ability and HBsAg release inhibition ability than single use of the antibody A3D5 and the antibody B5H6, and has strong synergistic effects, so the bispecific antibody is likely to prevent HBV infection related hepatitis, hepatic cirrhosis and liver cancer. The bispecific antibody is a completely humanized antibody obtained through cloning HBsAg specific memory B cells in the peripheral blood of hepatitis B vaccine inoculated volunteer, has lower immunogenicity than murine, chimeric and humanized antibodies, and can be used to prepare hepatitis B virus related hepatopathy prevention or treatment drugs or diagnose reagents.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
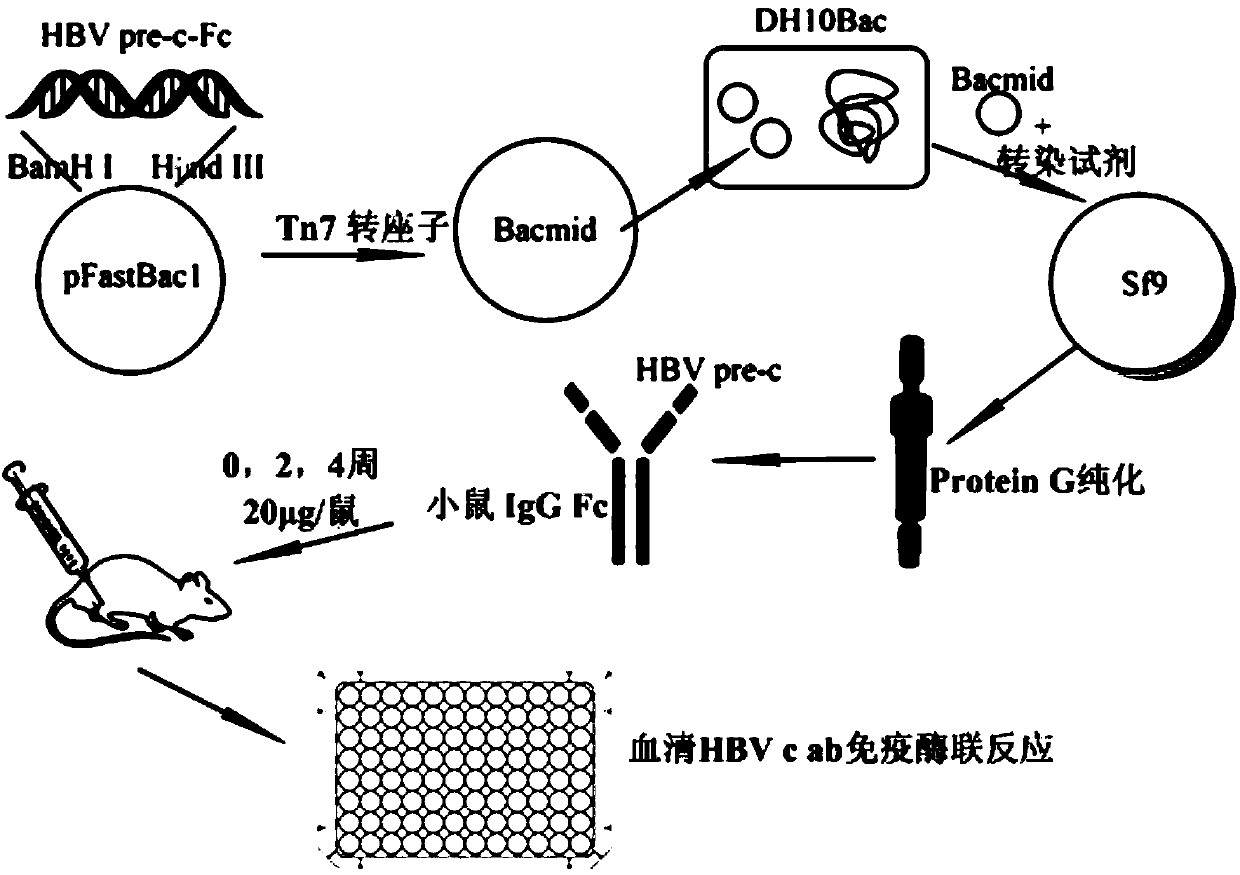
Recombinant protein and expressing method thereof in insect baculovirus expression system
InactiveCN104829732AEffective generationGenerating effective stimuliAntiviralsPharmaceutical non-active ingredientsBALB/cAntigen
The invention discloses HBV pre-c-Fc recombinant protein and a coding nucleotide sequence thereof and discloses a recombinant vector containing a coding sequence of the protein and a host cell. The invention further discloses a recombinant baculovirus, a method of obtaining the recombinant baculovirus, and a method of expressing the HBV pre-c-Fc recombinant protein in an insect baculovirus expression system, and also discloses a recombinant baculovirus containing a HBV pre-c-Fc coding gene and applications of the HBV pre-c-Fc recombinant protein in preparation of vaccines or medicines preventing or treating hepatitis B. After a BALB / c mouse is subjected to injection immunization with the HBV pre-c-Fc recombinant protein, detection shows that the generating amount of a hepatitis B virus core protein antibody in serum of the mouse is far higher than that in a situation that an antigen used for preparing hepatitis B vaccines in traditional methods is used.
Owner:WENZHOU MEDICAL UNIV
Hepatitis B vaccine preparation method using aluminium phosphate adjuvant in-situ method
ActiveCN103330936AUniform particlesFine particleAntiviralsAntibody medical ingredientsAntigenAdjuvant
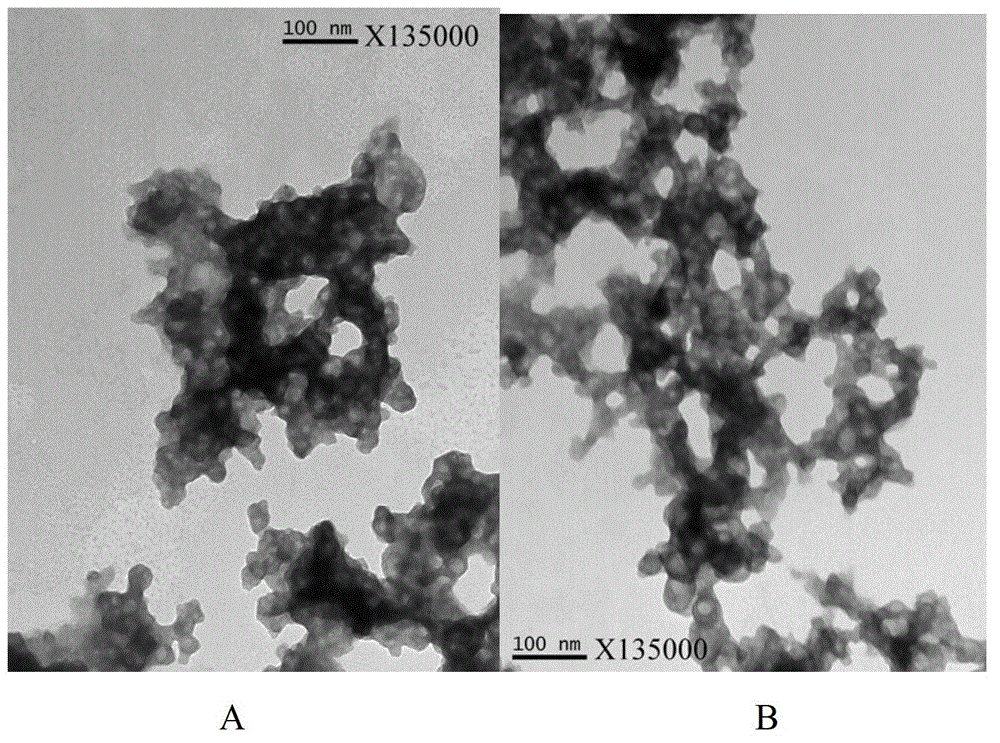
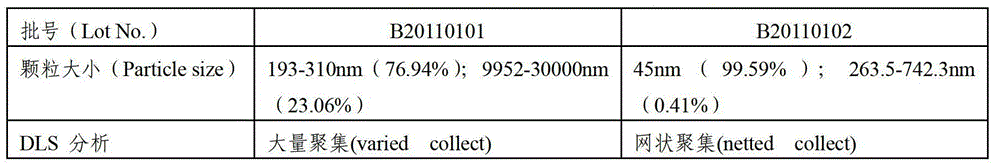
The invention relates to a hepatitis B vaccine preparation method using an aluminium phosphate adjuvant in-situ method. The hepatitis B vaccine preparation method comprises the following steps: 1) sequentially adding an aluminum chloride solution, water, a sodium chloride solution and hepatitis B surface antigen stoste into a reactor for stirring and mixing; 2) adding a mixing solution of disodium hydrogen phosphate and sodium hydroxide for stirring. The vaccine prepared through the method is a polydispersity system with a grain diameter about 45 nm, the grains of the vaccine are uniform, fine and smooth, and the vaccine has excellent intracorporal and extracorporeal relative potencies and high stability. The hepatitis B vaccine preparation method is short in production cycle, simple in technological operation and low in manufacturing cost, and facilitates large-scale production.
Owner:BEIJING MINHAI BIOTECH
Human anti-HBV surface antigen genetic engineering antibody, and preparation method and application thereof
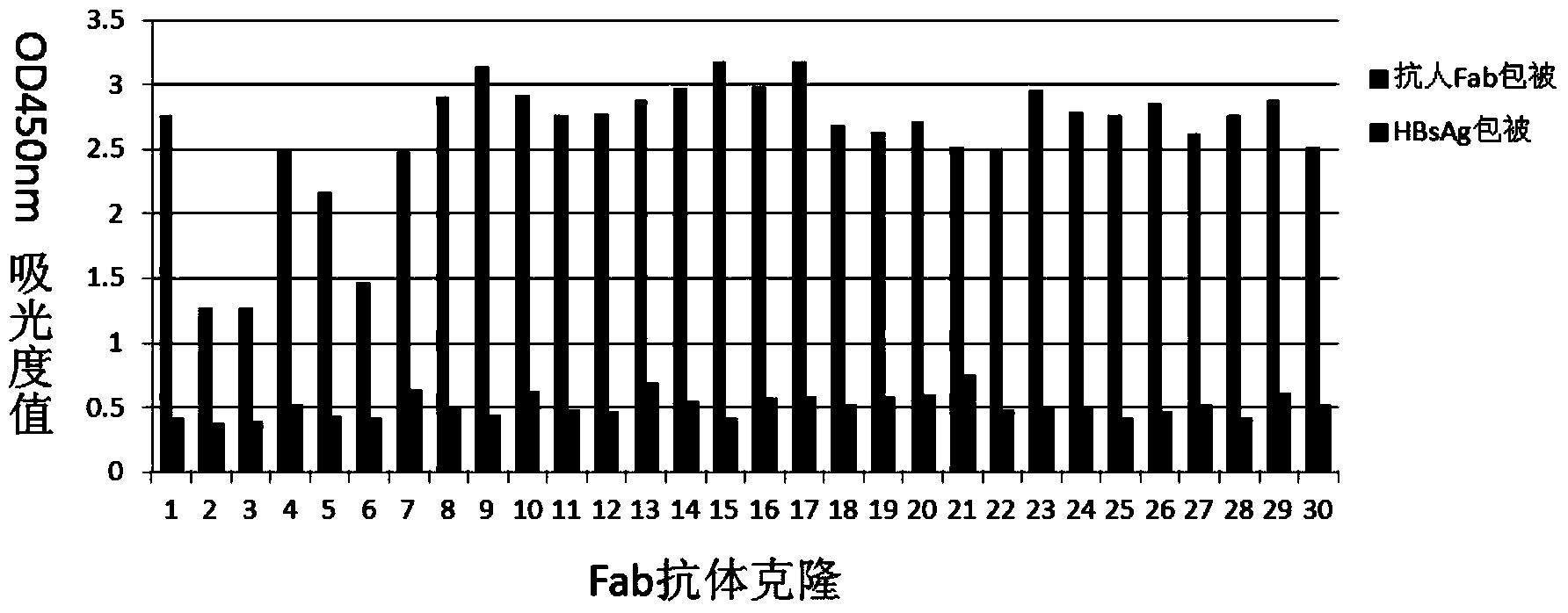
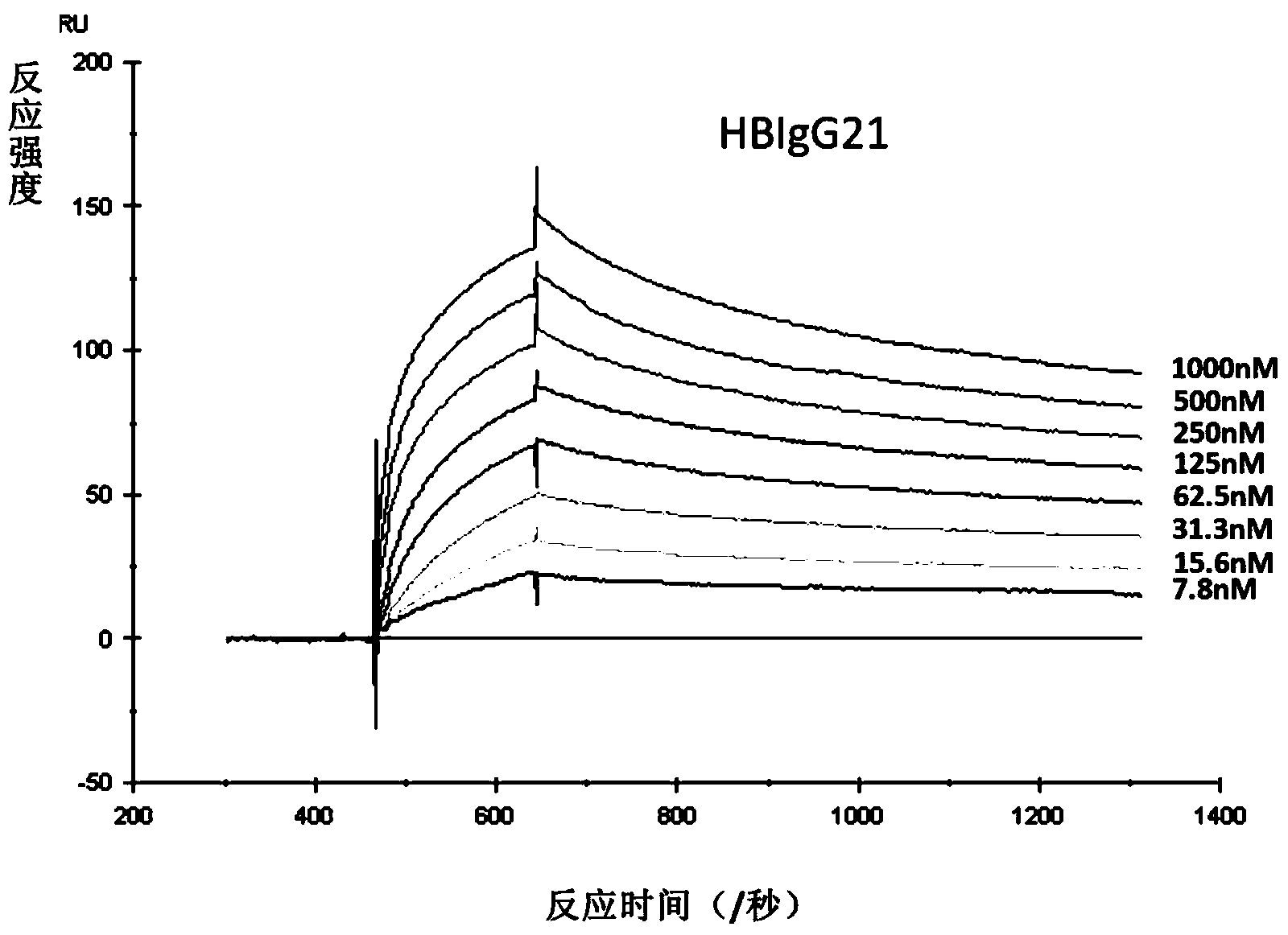
The invention discloses a human anti-HBV surface antigen genetic engineering antibody, and a preparation method and application thereof. According to the invention, the phage surface display technology is adopted, peripheral blood lymphocyte of person having high titer surface-antibody after immunization of hepatitis B vaccine is collected is obtained, a human anti-HBV surface antigen genetic engineering antibody library is established through a genetic engineering means, and a Fab section of the specific anti-HBV surface antigen genetic engineering antibody is obtained through screening. The obtained Fab section of the antibody is named to be HBFab21. The amino acid sequence of a light chain variable region of the HBFab21 is shown in SEQ ID No.1, and the amino acid sequence of a heavy chain variable region of the HBFab21 is shown in SEQ ID No.2. According to the invention, a foundation for hepatitis B virus infection prevention and researches on treatment to hepatitis B virus is laid; through the foundation, an antibody product having an effect of neutralizing hepatitis B virus infection is obtained, and can be applied to preparation of a clinical drug or diagnostic reagent for liver diseases relating to hepatitis B virus.
Owner:中国疾病预防控制中心病毒病预防控制所
B-type hepatitis vaccine
InactiveCN1404875ABright future for treatmentImprove humoral immunity/cellular immunityDigestive systemAntiviralsAdjuvantChronic hepatitis
The present invention relates to a hepatitis B vaccine, the main composition of said vaccine comprises gene engineering hepatitis B virus surface antigen, muramyl dipeptide (MDP) and aluminium adjuvant. Said hepatitis B vaccine can be used for immunity of adult, renal transplanted patient and patient with renal dialysis therapy and for preventing infection of hepatitis B virus, and said hepatitis B vaccine also can be used for immunotherapy of chronic hepatitis B patient, and the immunogenicity of said vaccine is superior to that of existent aluminium adjuvant hepatitis B vaccine.
Owner:BEIJING LUZHU BIOTECH
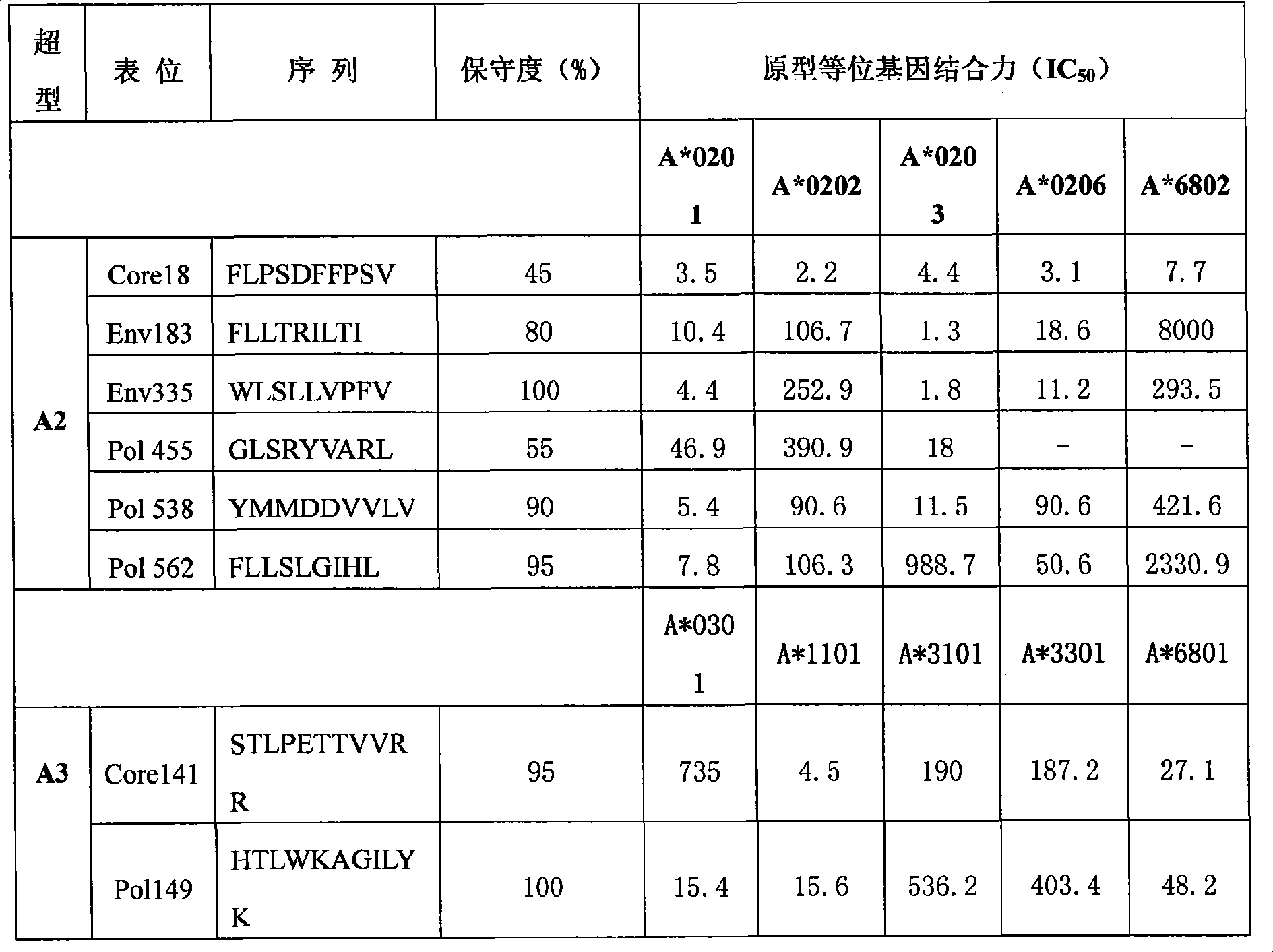
Hepatitis B virus multi-epitope fusion protein and preparation method and application thereof
ActiveCN102199217AHighly inhibitoryEfficient removalDigestive systemAntiviralsEscherichia coliFusion Protein Expression
The invention relates to a hepatitis B virus multi-epitope fusion protein and a preparation method and application thereof. The fusion protein is obtained by inserting hepatitis B virus multi-epitope fusion peptide (with the sequence shown as SEQIDNo.1) formed by serially connecting HBsAg313-321, HBsAg335-343, Pol150-159, Pol455-463 and Padre epitopes through connecting peptide between amino acidat the 78th position and amino acid at the 79th position of a hepatitis B virus core protein; and the preparation method comprises the following steps of: constructing a hepatitis B virus multi-epitope fusion protein expression plasmid pET28-HBc-HP; performing isopropyl thiogalactoside (IPTG) inducing expression by using an Escherichia coli expression system; and purifying by using affinity chromatography. The fusion protein carries a plurality of supertype epitopes of hepatitis B surface antigen (HBsAg), hepatitis B core antigen (HBcAg) and polymerase, is viral particles, has the advantages of strong immunogenicity, wide applicable range and the like, and can be used for preparing therapeutic hepatitis B vaccines.
Owner:ARMY MEDICAL UNIV
Application of hepatitis B surface antigen-antibody complexes in preparing prophylaxis product with no response or low response to hepatitis B vaccine
ActiveCN1919341AEffective infectionProtection from infectionDigestive systemAntiviralsSerum igeHepatitis A vaccine
The invention discloses a new utility of hepatitis B surface antigen-antibody compound in the hepatitis B vaccine non-respond or low-respond prevention agent, which is characterized by the following: purifying recombined HBsAG or inactivated serum HBsAG in the mammal cell, adopting hepatitis B with antibody serum or vaccine globulin as compound, improving anti-HBs ability for non-respond or low-respond mouse.
Owner:FUDAN UNIV +1
Immuno liposome therapeutic hepatitis B vaccine and its preparing method
InactiveCN1364644AGood prevention effectTrigger immune responseDigestive systemAntiviralsFreeze-dryingHepatitis B Surface Antigens
The therapeutic hepatitis B vaccine is liposome containing hepatitis B surface antigen with or without immunoregulation molecule. It may be used to treat chronic hepatitis B caused by HIV infection and to prevent HIV infection. The technological process of inverse phase evaporation and freeze drying to prepare the said vaccine is also provided.
Owner:DOMINO MEDICINE INST BEIJING
Preparation method of hepatitis vaccine and its use
InactiveCN1824304AReduction in inflammatory nodulesLittle side effectsPowder deliveryDigestive systemAdjuvantInjection site
A human rabies vaccine is prepared from the existing hepatitis B vaccine without adjuvant through adsorbing it by the aluminum hydroxide nanoparticles. Its adsorbed capacity is increased by 10-20 time. It can be used for emergency inoculation for preventing hepatitis B as it has the fast release effect.
Owner:THE THIRD AFFILIATED HOSPITAL OF THIRD MILITARY MEDICAL UNIV OF PLA
Hepatitis B recombinant antigen, expression gene of hepatitis B recombinant antigen, construction method of hepatitis B recombinant antigen, virus-like particle of hepatitis B recombinant antigen, preparation method of virus-like particle of hepatitis B recombinant antigen, applications of hepatitis B recombinant antigen, and vaccine
ActiveCN106928372APrevent infectionPlay a role in clearingDigestive systemInactivation/attenuationHemagglutininNucleotide
The invention relates to a hepatitis B recombinant antigen, an expression gene of the hepatitis B recombinant antigen, a construction method of the hepatitis B recombinant antigen, a virus-like particle of the hepatitis B recombinant antigen, a preparation method of the virus-like particle of the hepatitis B recombinant antigen, applications of the hepatitis B recombinant antigen, and a vaccine. The hepatitis B recombinant antigen is obtained by expressing a polynucleotide comprising a nucleotide sequence shown in the SEQ ID NO.1. The expression gene of the hepatitis B recombinant antigen is obtained by splicing a preS region of the hepatitis B virus with a signal peptide region of an influenza virus hemagglutinin and splicing a transmembrane region of the influenza virus with an intracellular region, the expression gene is successfully expressed to obtain the hepatitis B recombinant antigen, and the hepatitis B recombinant antigen is better in immunogenicity, and can enable the immune system of the minority (about 5%-10%) incapable of producing antibodies for the traditional vaccine to make an immune response. Meanwhile, on the basis of the indispensable importance of the preS region for the hepatitis B virus, the vaccine for broader intended population is prepared for the preS region. The vaccine has the effects of preventing infection of the hepatitis B virus and clearing away the hepatitis B virus, and is expected to be transformed into a novel preventive and therapeutic hepatitis B vaccine on medical science.
Owner:PEKING UNIV SHENZHEN GRADUATE SCHOOL
Vaccine composition for treating B-type hepatitis and preparing method
This invention discloses a new style curing hepatitis B combination and its preparation method; it belongs to biological agent field. The vaccine pharmaceutics carries recombination yeast hepatitis B and human recombination IL-l2, their weight ratio is 60-80:2-10. the preparation method is that recombination yeast hepatitis B and L-l2, or recombination yeast hepatitis B and IL-l2 and IL-2 are directly mixed to make hepatitis B vaccine combination, pharmaceutics is water agent. This invention has better therapeutic effect, and is used for hepatitis B curing.
Owner:广州市恺泰生物科技有限公司
Hepatitis B nucleic acid vaccine and construction method thereof
InactiveCN101954093AImprove protectionGood immune effectGenetic material ingredientsDigestive systemTreatment effectHepatitis B virus DNA
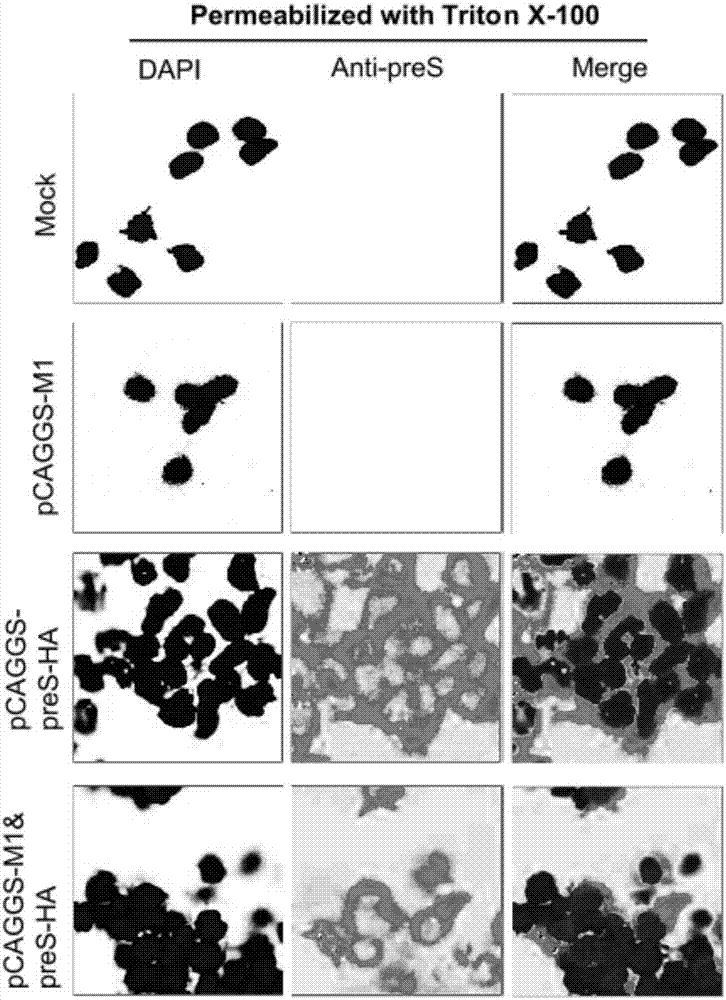
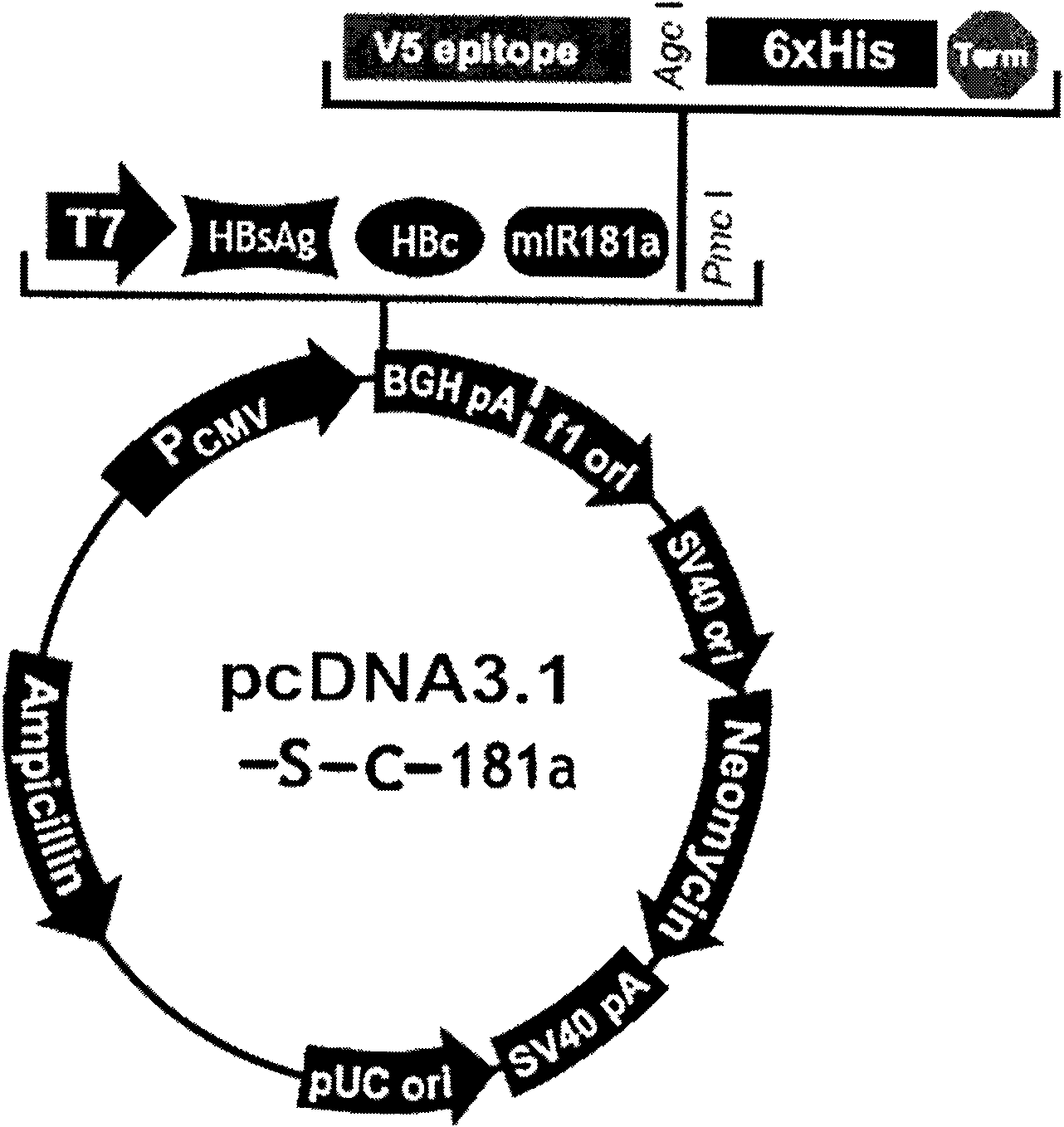
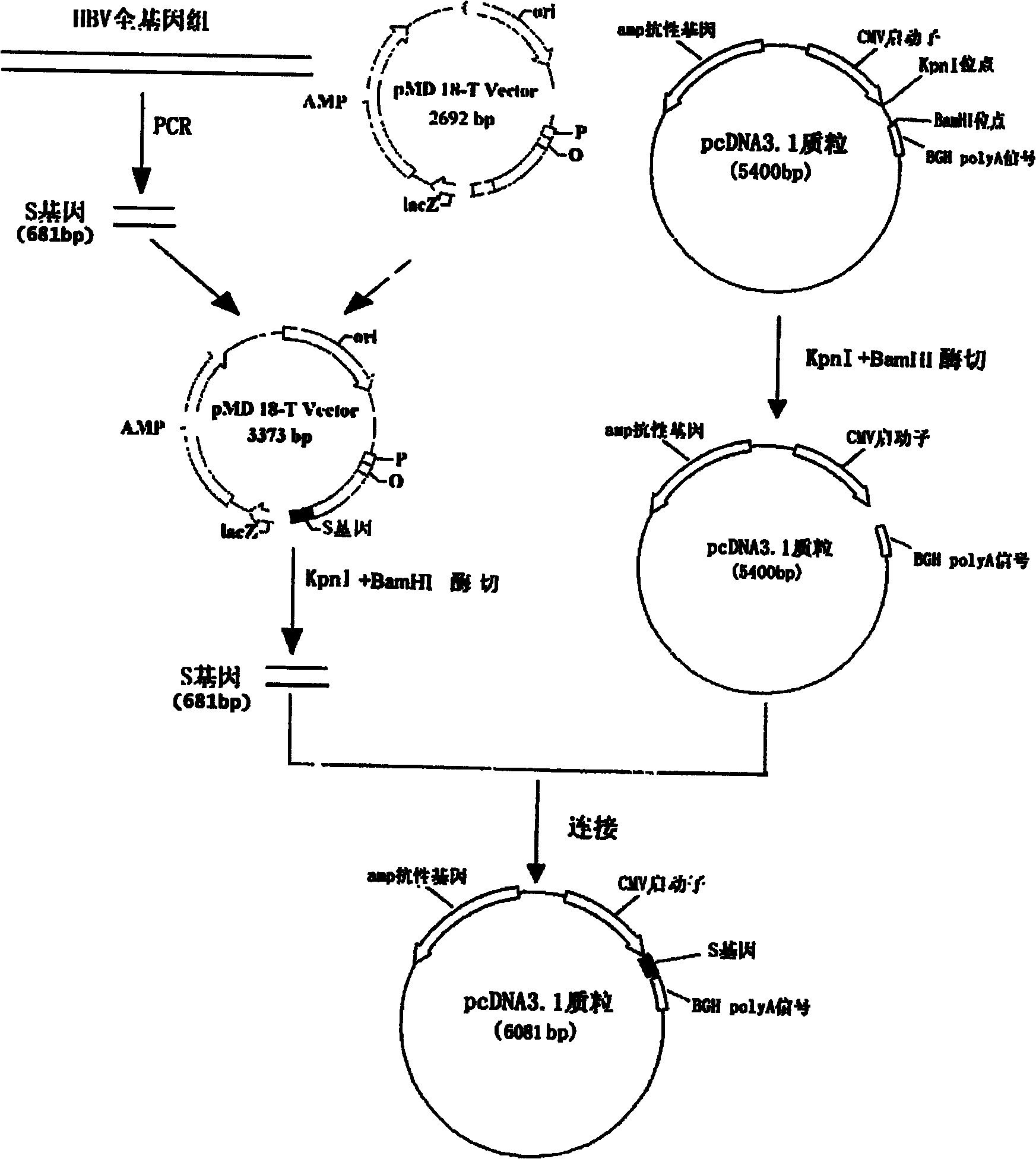
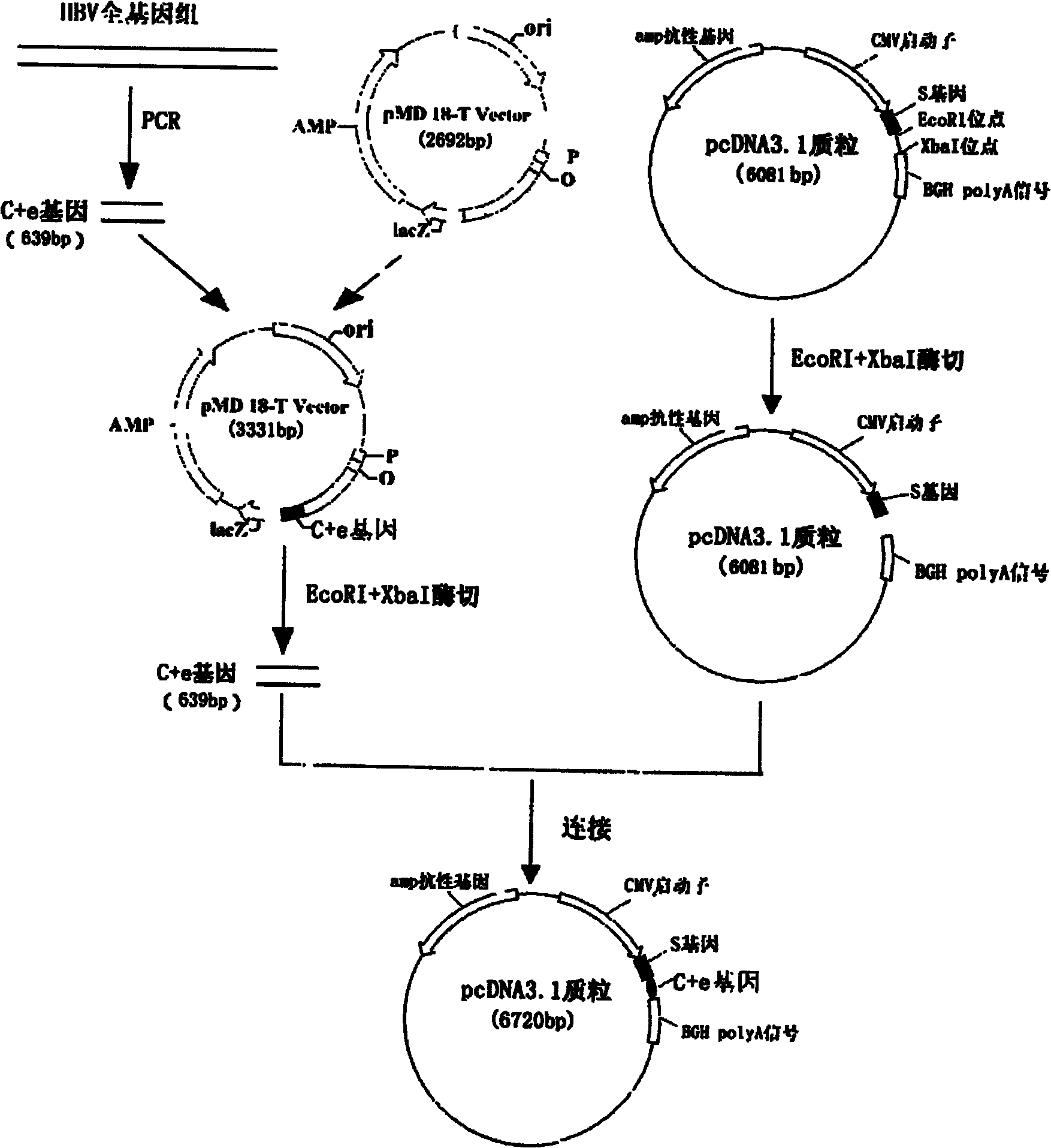
The invention relates to the technical field of biomedicines. The currently reported hepatitis B virus vaccine mainly comprises one or several of HBsAg, preS 1, preS2 and HBcAg, some cell factor genes with immunological enhancement function or lymphocyte epitope genes and the like, however, the immunoprophylaxis effect and treatment effect of the vaccine are always unsatisfactory. The invention provides a hepatitis B vaccine which comprises hepatitis B virus surface antigen gene HBsAg, core protein gene HBc and e-antigen gene, also comprises a human microRNA181a precursor gene sequence, and can assist stimulating the body immunity pathway. The construction process of the vaccine relates to PCR, enzyme cutting, connection, conversion and other molecularly biological operating means. The hepatitis B vaccine has the advantages of effectively activating the body to produce a specific antibody against the hepatitis B virus, stimulating body cell immunity and secreting various cell factors. Therefore, the aim of treating chronic hepatitis B is fulfilled.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Hepatitis B vaccine for inducing organism to generate specific immunity in state of chronic hepatitis B virus infection
The invention discloses a hepatitis B vaccine, and in particular discloses a hepatitis B vaccine which contains an immunopotentiator and can induce an organism to generate specific immunity in a state of chronic hepatitis B virus infection. The hepatitis B vaccine disclosed by the invention contains a Toll-like receptor agonist serving as the immunopotentiator.
Owner:李金秋
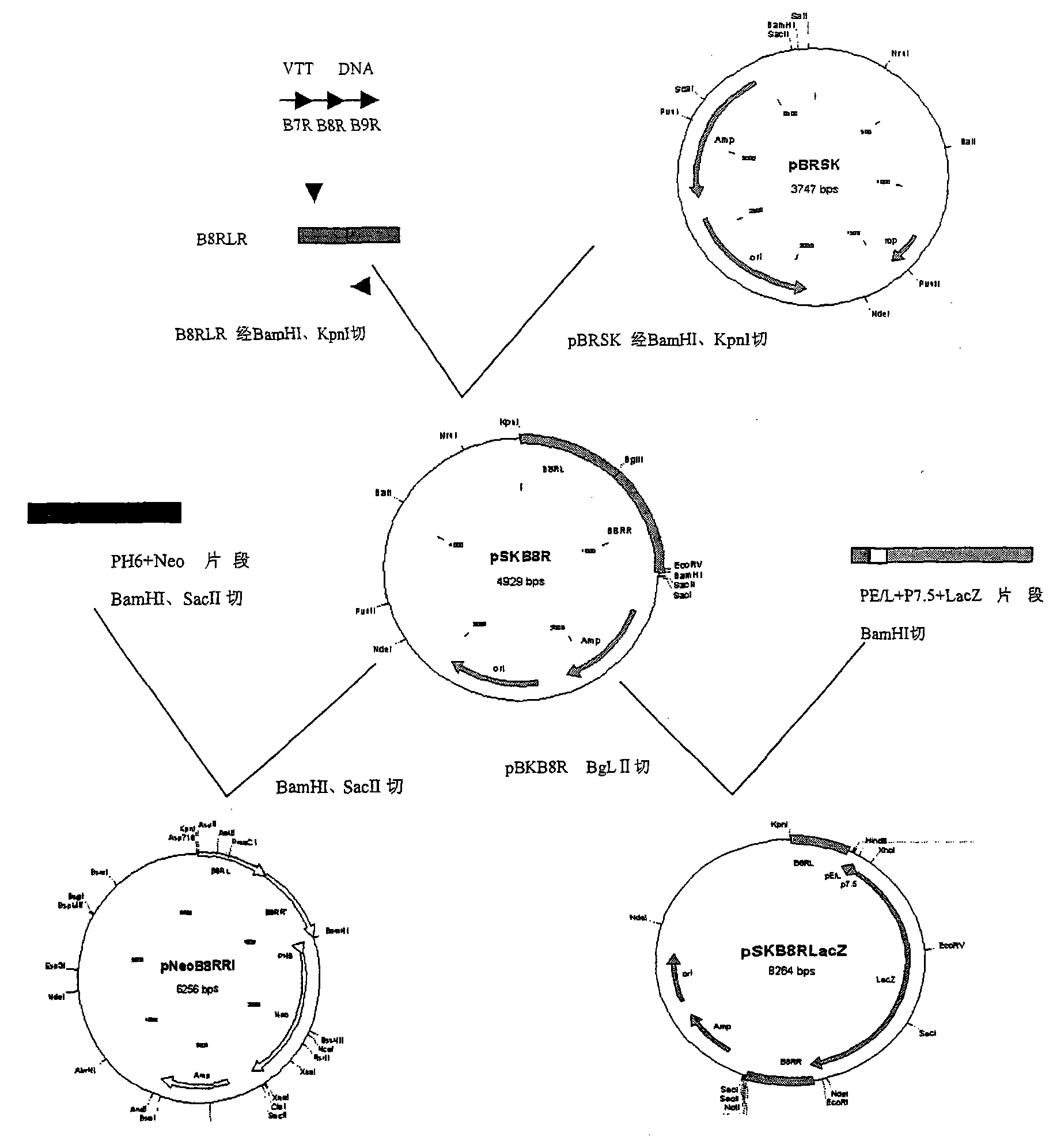
Recombinant vaccinia virus of Tiantan strain with IFN-gamma receptor gene (B8R) deletion and applications thereof
InactiveCN101671695ALow toxicityImprove securityMicroorganism based processesAntiviralsAntigenViral Vaccine
The invention relates to an attenuated carrier of recombinant vaccinia virus of Tiantan strain with IFN-gamma receptor gene (B8R) deletion, and AIDS vaccines of recombinant vaccinia virus of Tiantan strain with IFN-gamma receptor gene (B8R) deletion used for expressing various antigens (univalent or polyvalent) of HIV-1 and constructed based on same, a recombinant vaccinia virius VTKgpe recombinant vacciniavirus VTKgpe CGMCC.NO.1099 and another hepatitis B virus HBSAg antigen, and hepatitis B virus vaccines of recombinant vaccinia virus of Tiantan strain of IL-2. The invention has an importantmeaning to the use for preparation of recombinant vaccinia virus vaccines for treating virus diseases.
Owner:NAT CENT FOR AIDSSTD CONTROL & PREVENTION CHINESE CENT FOR DISEASE CONTROL & PREVENTION
Recombinant influenza A virus carrying hepatitis B virus gene, host cell, preparation method for recombinant influenza A virus and application of recombinant influenza A virus
InactiveCN111363728AImprove shear efficiencyCut effectivelySsRNA viruses negative-senseViral antigen ingredientsHepatitis B immunizationEmbryo
The invention discloses a recombinant influenza A virus carrying a hepatitis B virus gene, a host cell, a preparation method for the recombinant influenza A virus and application of the recombinant influenza A virus. According to the recombinant influenza A virus disclosed by the invention, by taking an influenza A virus as a vector, and by utilizing a reverse genetics technique, the hepatitis B virus gene is integrated onto an influenza virus genome, and thus, the recombinant influenza virus can passage steadily in the host cell or a chicken embryo. The recombinant influenza A virus can be used for development of hepatitis B vaccines, development of related drugs, and used for producing hepatitis B associated proteins by utilizing the chicken embryo or the cell as a bioreactor.
Owner:WUHAN UNIV
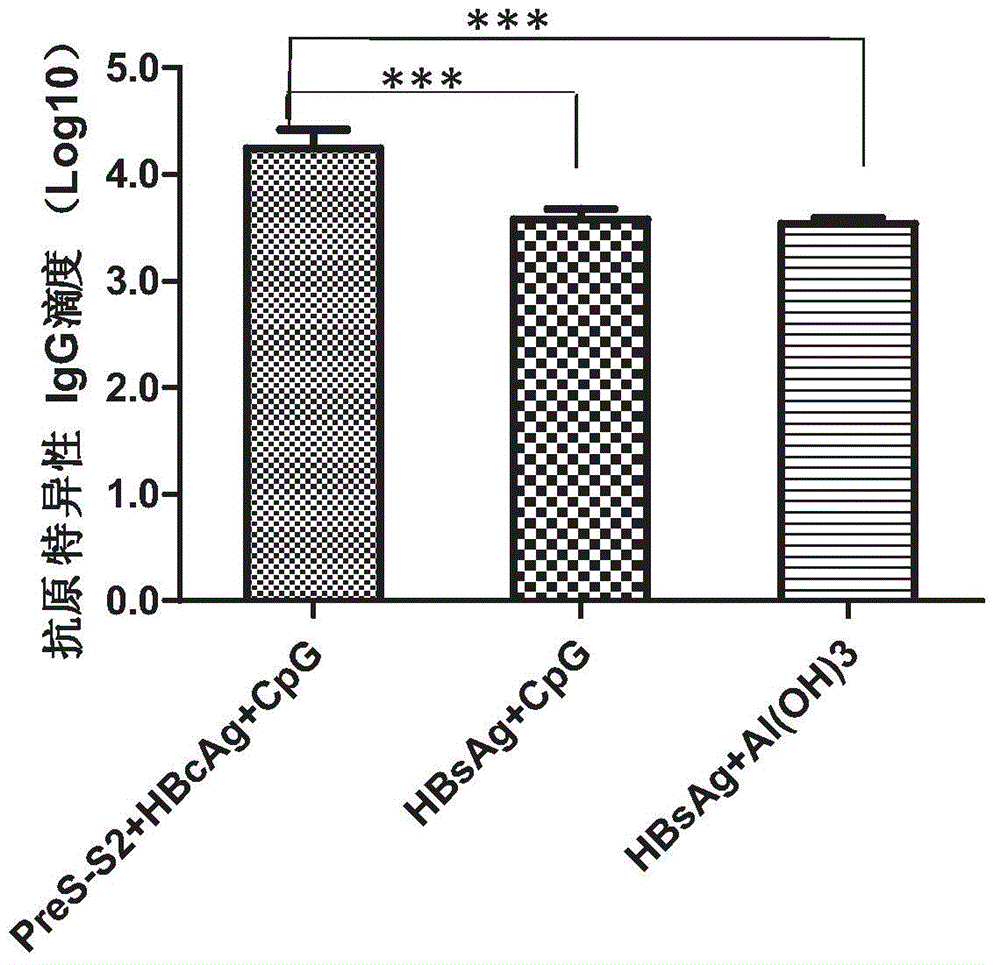
Therapeutic hepatitis B vaccine based on HBV PreS-S, C antigen and novel adjuvant CpG
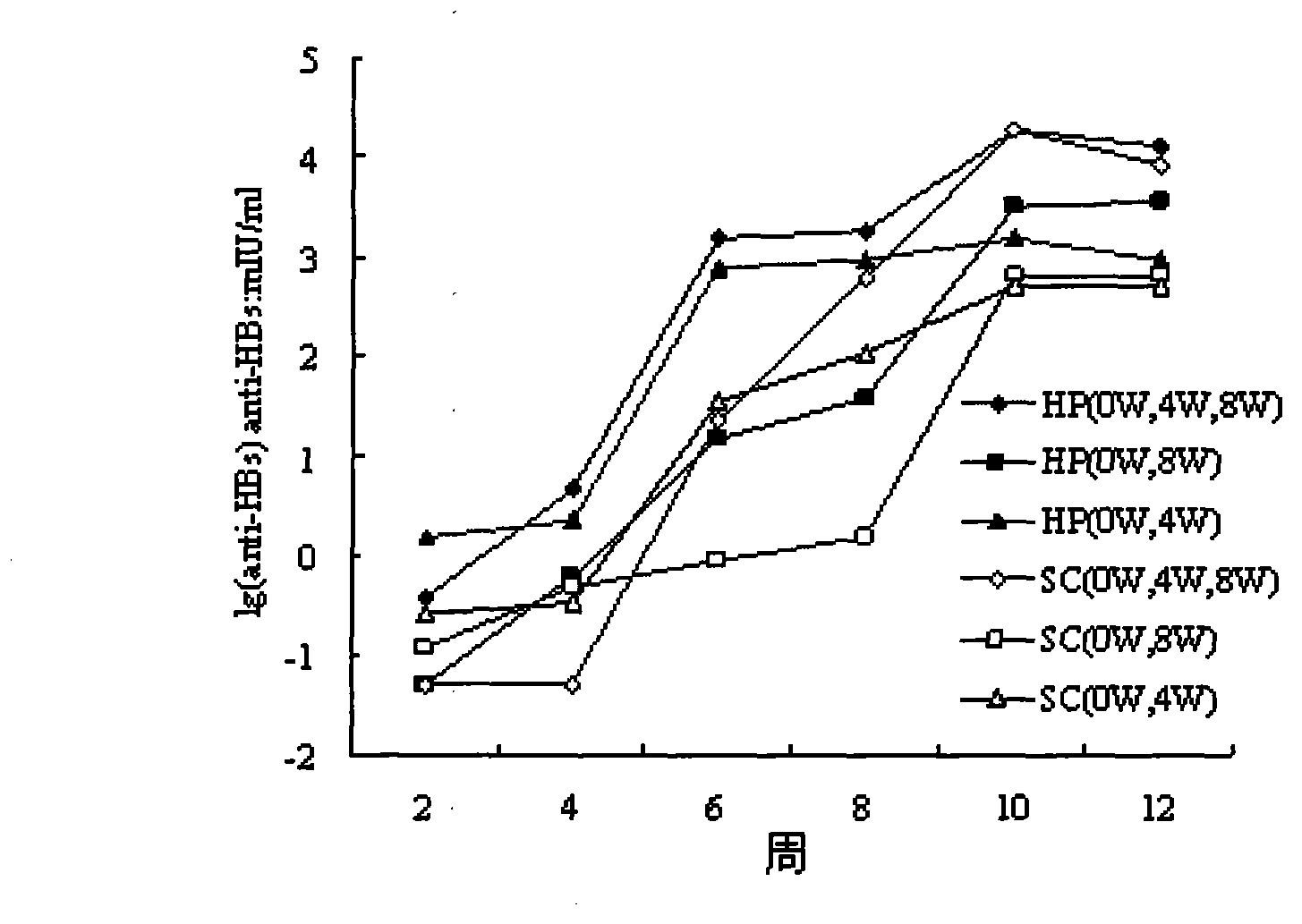
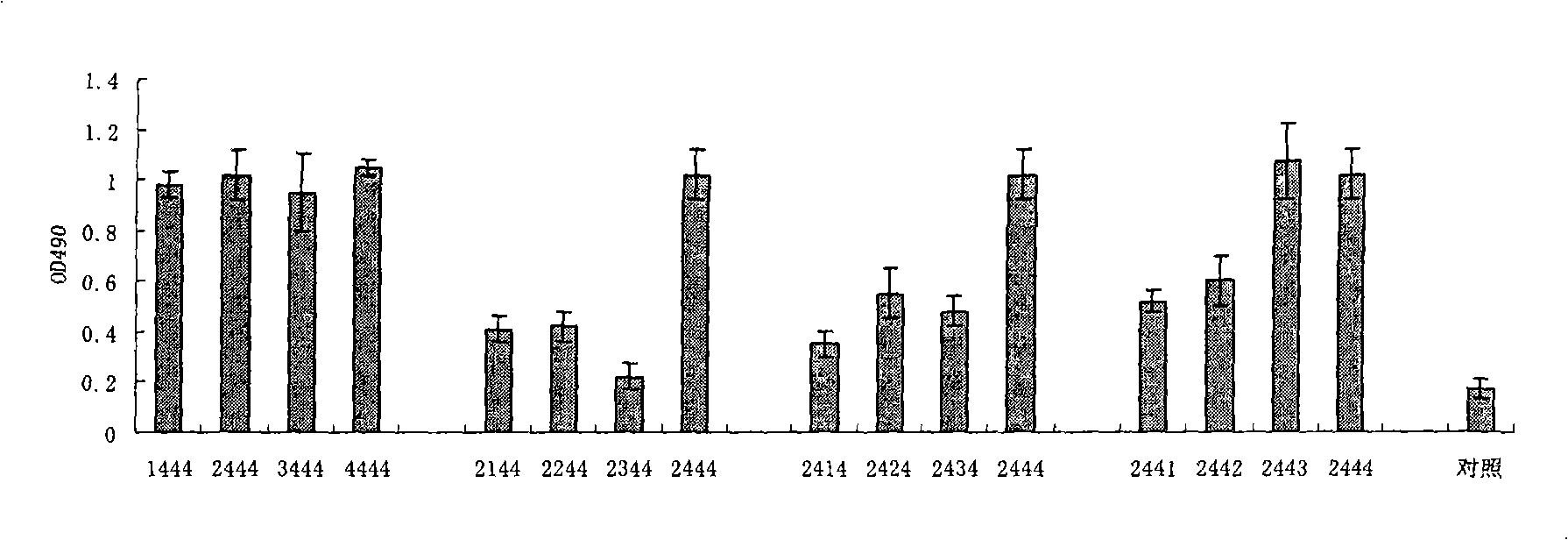
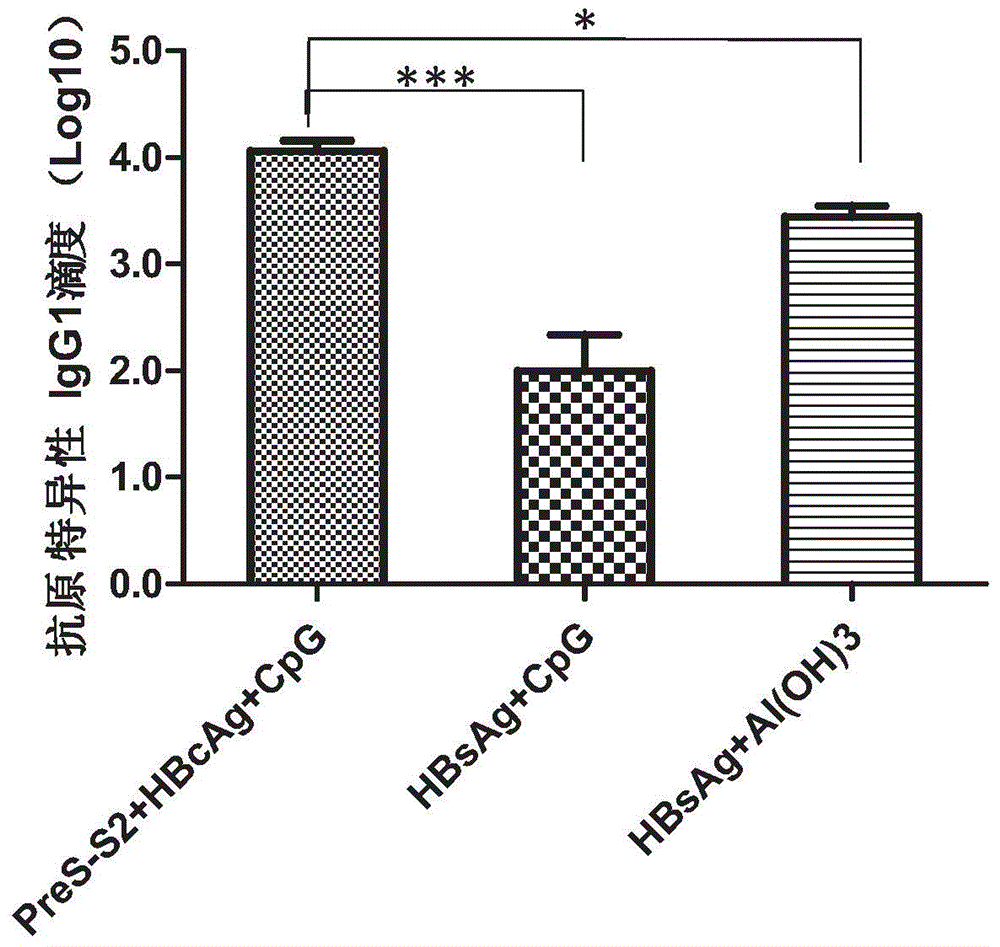
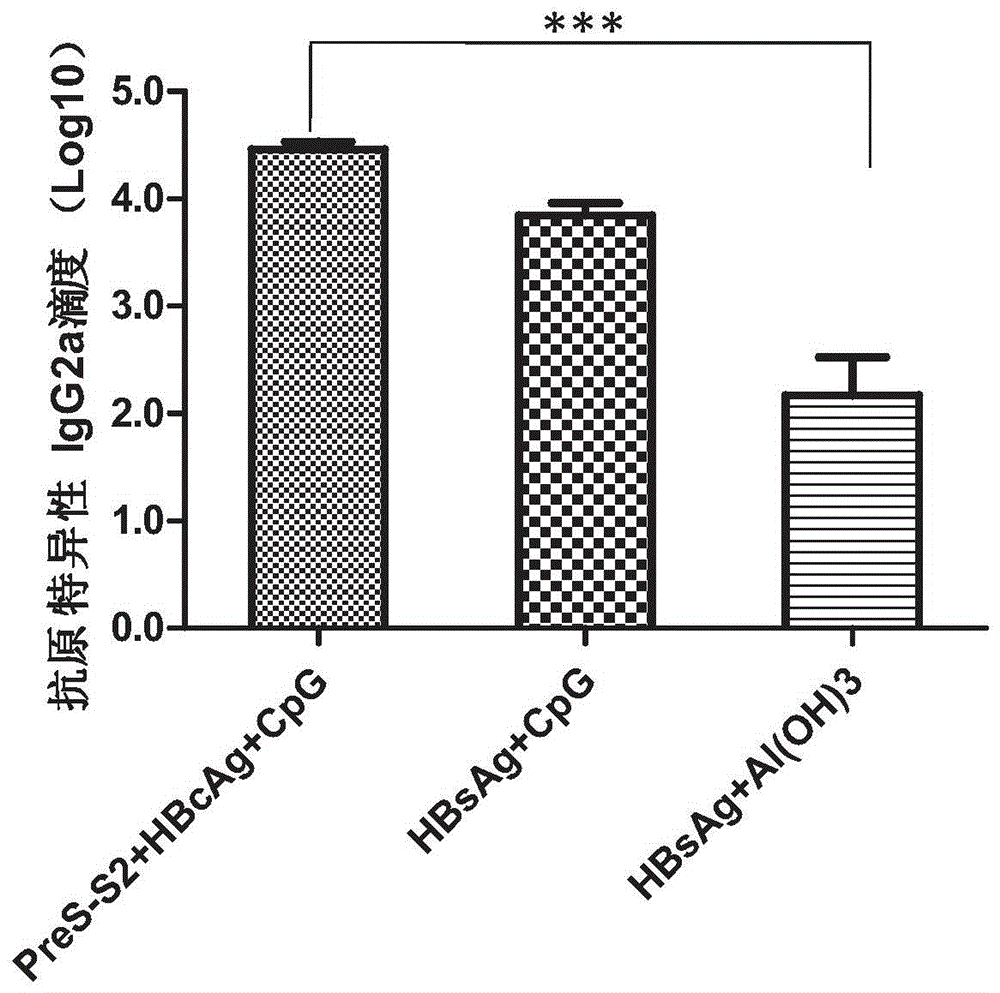
The invention relates to a composition. The composition comprises (i) HBsAg and a precursor thereof (PreS-S), a fragment of the antigen, a variant of the antigen or a mixture of at least two of the HBsAg and the precursor thereof (PreS-S), the fragment and the variant; (ii) HBcAg1-X, a fragment of the antigen, a variant of the antigen or the fragment, or a mixture of at least two of HBcAg1-X, the fragment and the variant, wherein X is an integral number from 149-183; (iii) CpG-ODN, the oligonucleotide is full sulpho-modified, the sequence has two or more copies of 5'-NTCGTT-3' motifs and the length is 21 basic groups. The invention also relates to an application of the composition for treating the HBV infected and HBV mediated diseases, and a method for treating the HBV infected and HBV mediated diseases.
Owner:JIANGSU THERAVAC BIO PHARMA
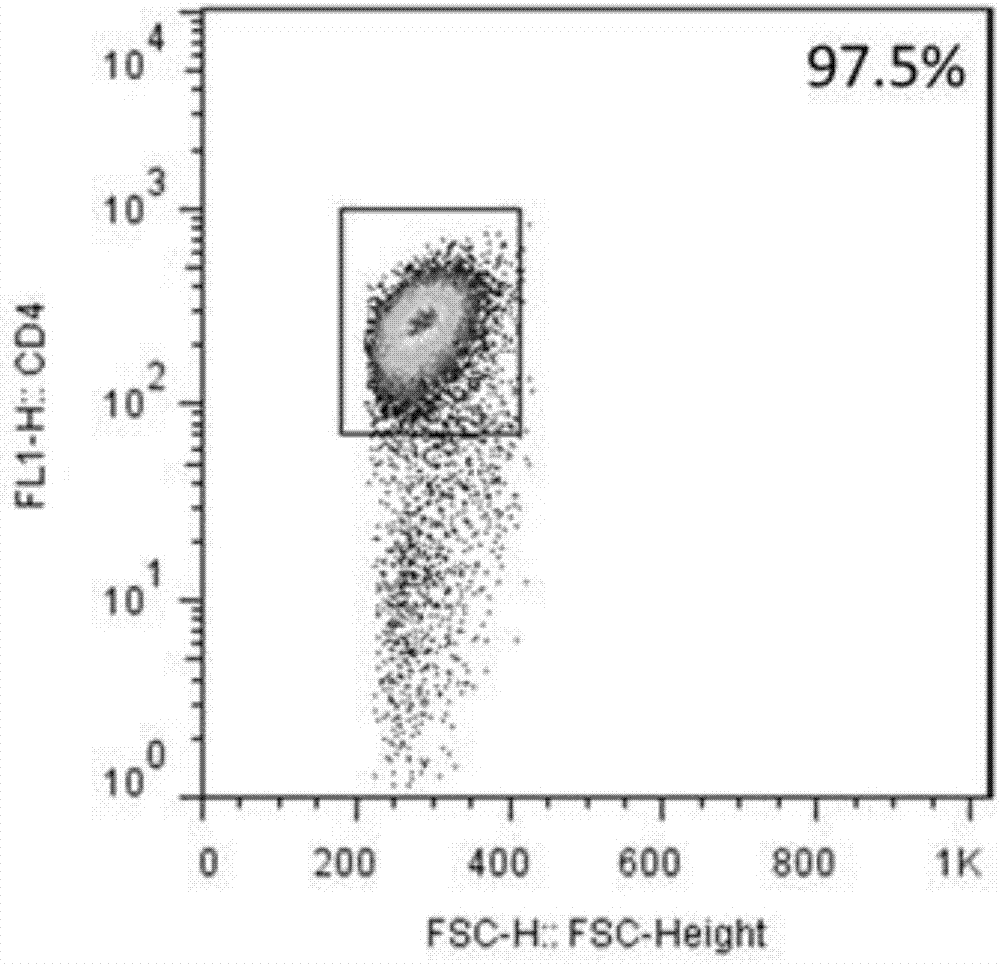
CD4+T cell derived exosomes and application thereof
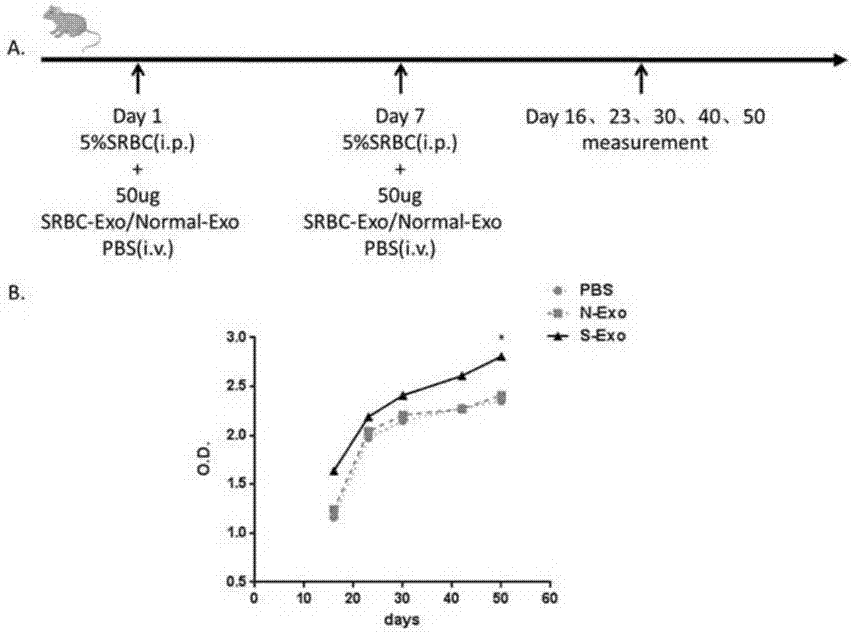
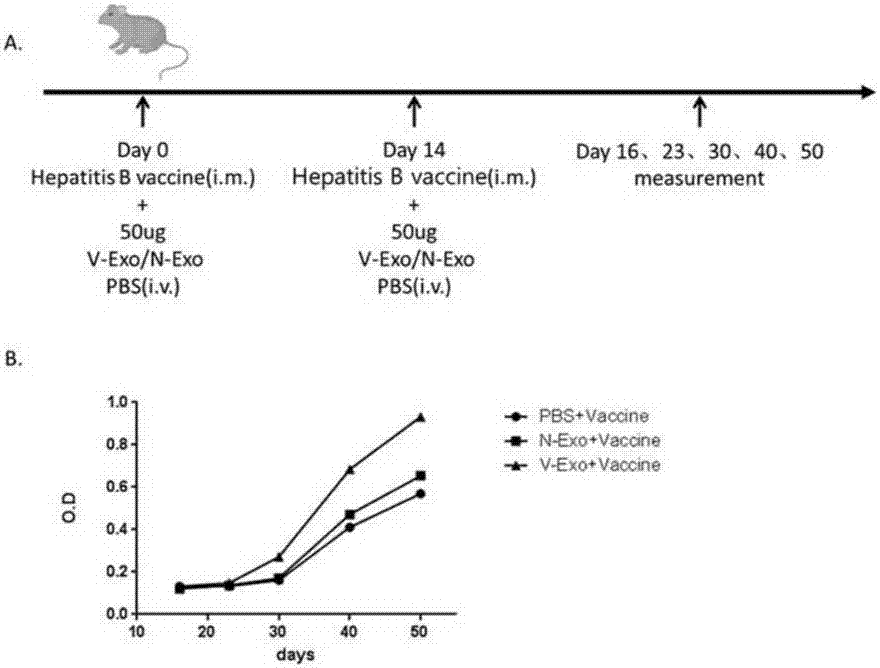
ActiveCN107365742APromote proliferationPromote productionViral antigen ingredientsDigestive systemImmune effectsAdjuvant
The invention realtes to CD4+T cell derived exosomes and application thereof, and belongs to the field of cytobiology, molecular biology and clinical application. The invention particularly discloses CD4+T cell derived exosomes (named as CD4+ T exo) and application thereof in preparation an adjuvant for enhancing a vaccine immunity effect. It discovers that CD4+ T exo can effectively promote proliferation and activation of B cells in vitro, and can promote the B cells to produce more antibodies. Meanwhile, CD4+ T exo can serve as an adjuvant to enhance the immune effect of sheep red blood cell (SRBC) immunized mice in vivo, can also have obvious enhancing effect on hepatitis B vaccine immunized Babl / c mice, and promotes peripheral blood to produce more hepatitis B surface antibodies (HbsAb). The application of the CD4+T cell derived exosomes can be used for preparing a novel adjuvant for enhancing the antigen / vaccine immunization effect.
Owner:JIANGSU UNIV
Hepatitis b vaccine and preparation technology thereof
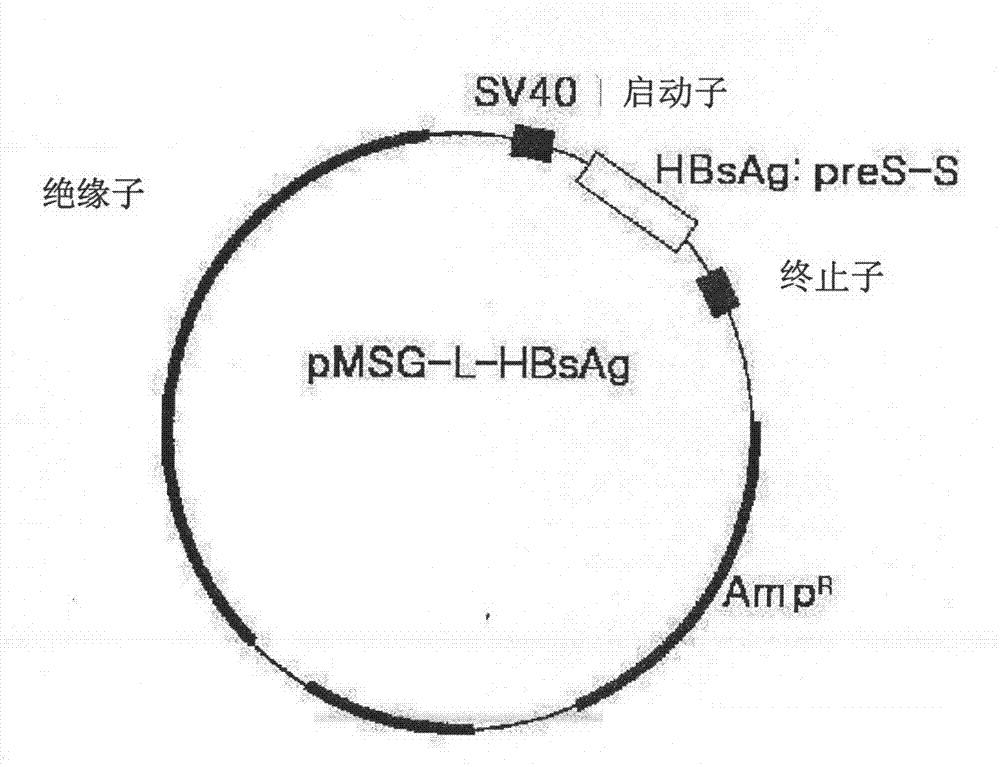
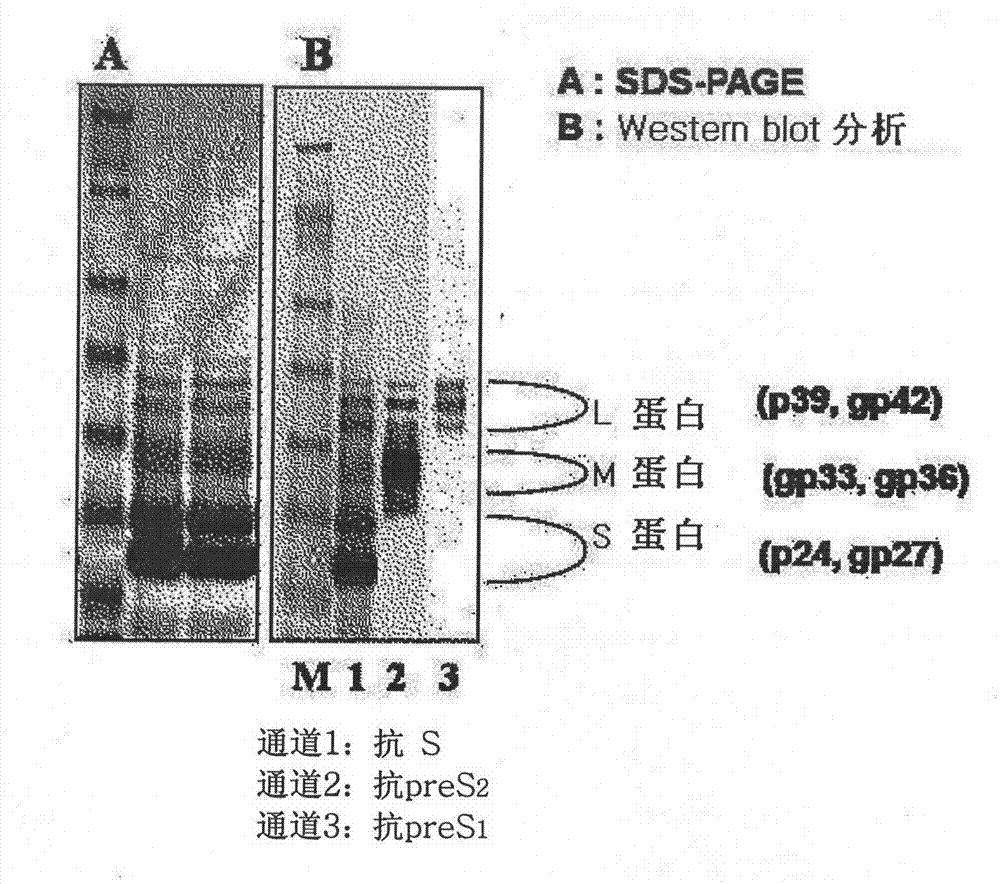
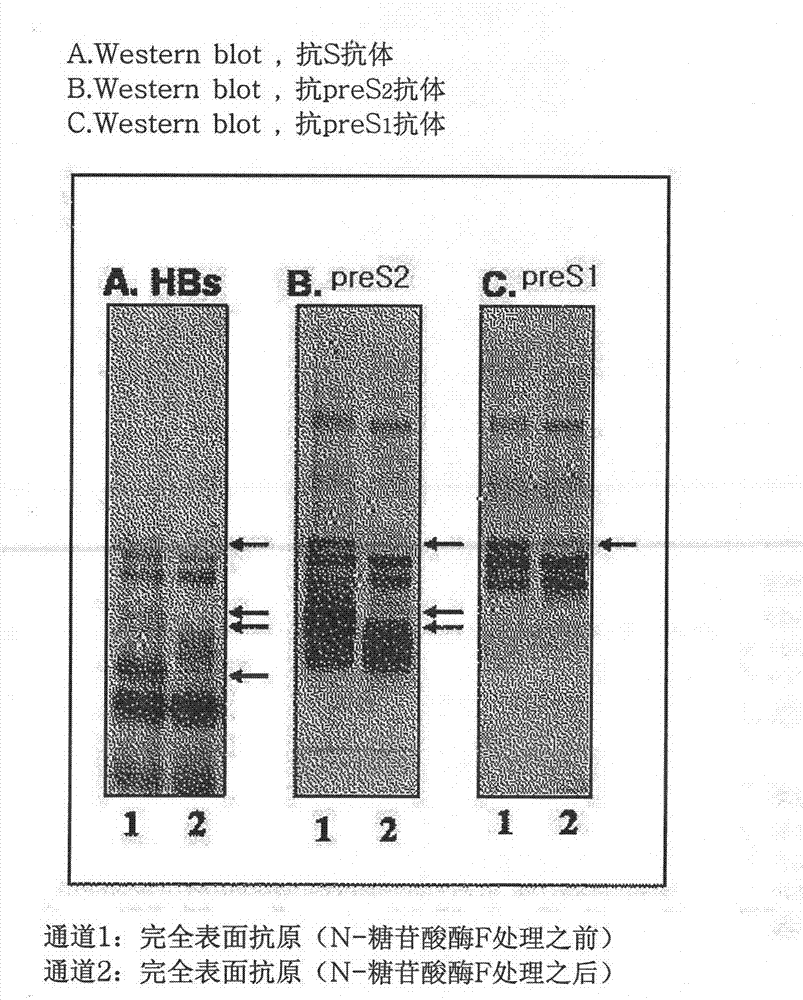
The invention discloses an expression vector expressing a recombinant complete HBV surface antigen comprising L protein, M protein and S protein, prepared by injecting a polynucleotide of a coding region which has a complete HBV envelope gene and a complete 3'-UTR nucleotide containing polyadenylation sites into a pSGM vector identified by the preservation number KCCM 10202.
Owner:DOBEEL CO LTD
Recombinant saccharomyces-fermentum-expressed hepatitis B surface antigen, production method of hepatitis B surface antigen, hepatitis B vaccine and production method of hepatitis B vaccine
ActiveCN103333938AComply with GMP requirementsHigh degree of automationDigestive systemVirus peptidesAntigenUltrafiltration
The invention discloses a production method of a recombinant saccharomyces-fermentum-expressed hepatitis B surface antigen. The method comprises the steps of: circularly removing triton from an extracted antigen by using XAD-4 column, so as to obtain triton-removed antigen sample liquid; loading macroporous silica gel with the pore size of 1,000 A and the particle size of 35-70 microns into a chromatographic column, and balancing by using phosphoric acid buffer solution with the pH of 7.6+ / -0.2; adjusting the pH of the triton-removed antigen sample liquid to be 7.6+ / -0.2 by using NaOH, then, loading a sample to the macroporous silica gel column; and cleaning impurities by using phosphoric acid buffer solution with the pH of 7.2+ / -0.2, carrying out eluting treatment on the impurity-cleaned macroporous silica gel column by using boric acid buffer solution with the pH of 8.7+ / -0.2 at the temperature of 47-49 DEG C, collecting eluate, and carrying out ultrafiltration and concentration on the eluate, thereby obtaining a clarified antigen. The invention further discloses the corresponding recombinant saccharomyces-fermentum-expressed hepatitis B surface antigen, a hepatitis B vaccine and a production method of the hepatitis B vaccine. The production methods have the advantages that the probability of product contamination is reduced greatly, the labor intensity for laborers is reduced, the equipment investment and repairing cost are reduced, the space occupied by equipment is reduced, and the production time is shortened.
Owner:SHENZHEN KANGTAI BIOLOGICAL PROD
Anti-hepatitis B virus surface antigen completely humanized human antibody and use thereof
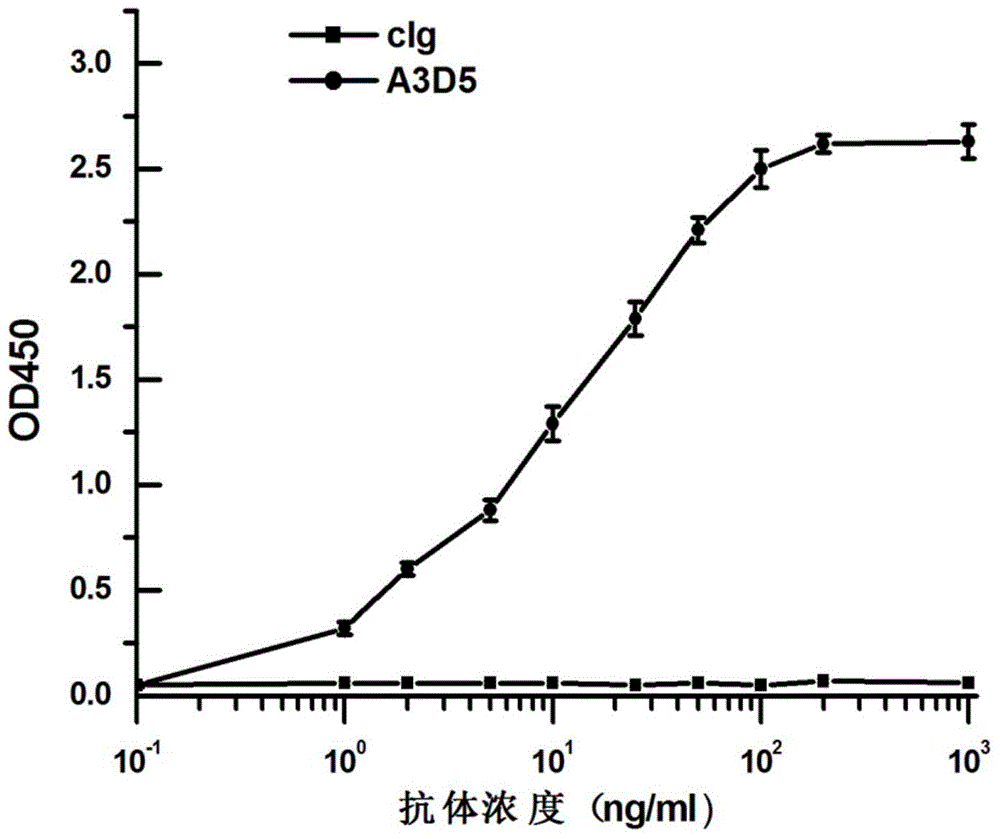
InactiveCN105061591ALow immunogenicityAvoid infectionImmunoglobulins against virusesAntiviralsHumanized antibodyFhit gene
The invention provides an anti-hepatitis B virus surface antigen completely humanized human antibody A3D5 and a gene for encoding the antibody. Experiments show that the antibody A3D5 can specifically bind to an HBsAg protein, and has good HBV neutralization activity in order to possibly prevent HBV infection related hepatitis, hepatic cirrhosis and liver cancer. The antibody is a completely humanized human antibody obtained through cloning HBsAg specific memory B cells in the peripheral blood of hepatitis B vaccine inoculated volunteer, has lower immunogenicity than murine, chimeric and humanized antibodies, and can be used to prepare hepatitis B virus related hepatopathy prevention or treatment drugs or diagnose reagents.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Sulpho-oligodeoxynucleotide with immunostimulation activities and application thereof
ActiveCN101979566AHigh activityEnhance immune responseOrganic active ingredientsAntiinfectivesNucleotideAllergy
The invention relates to sulpho-oligodeoxynucleotide with immunostimulation activities. The sequence of the sulpho-oligodeoxynucleotide has two or more than two copied 5'-NTCGTT-3' elements having 15 to 35 nucleotides respectively, wherein CpG is non-methylated and N does not represent A or G. In vitro, the sulpho-oligodeoxynucleotide has high immunostimulation activities for the immunocytes of human and a mouse, and the immunostimulation activities of the sulpho-oligodeoxynucleotide in a human body can be evaluated according to the result of the immunostimulation activities in mouse. Used asa vaccine adjuvant, the sulpho-oligodeoxynucleotide can obviously strengthen the immune response of the mouse to a genetic engineering hepatitis B vaccine and make the immune response deflected to a TH1 direction, and thus, it is proved that the sulpho-oligodeoxynucleotide can be used for preventing and treating allergy and infection. Meanwhile, the sulpho-oligodeoxynucleotide has high tumor growth restraining activities in the mouse body.
Owner:苏州博特龙免疫技术有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com