Hepatitis b vaccine and preparation technology thereof
A hepatitis B and vaccine technology, applied in medical preparations containing active ingredients, viral peptides, cells modified by introducing foreign genetic material, etc., can solve the problem that preS antigen is not very immunogenic
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0085] Hereinafter, the present invention will be described in more detail with examples. However, these examples are for illustrative purposes only, and the present invention is not intended to be limited to these examples.
example 1
[0086] The preparation of example 1 hepatitis B vaccine
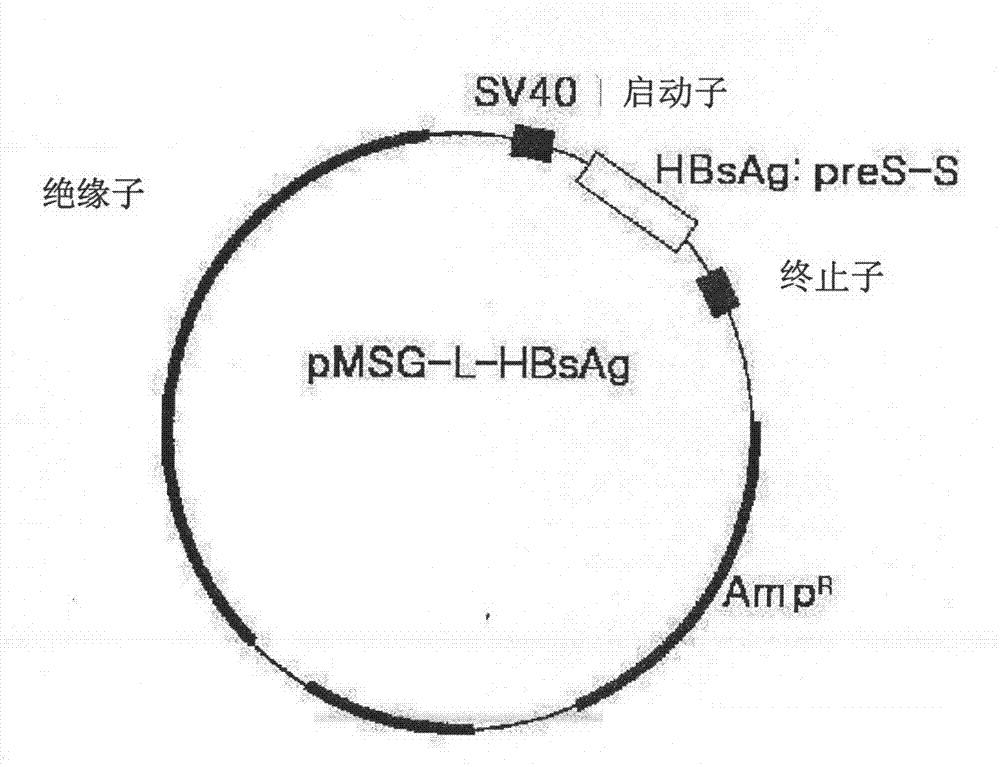
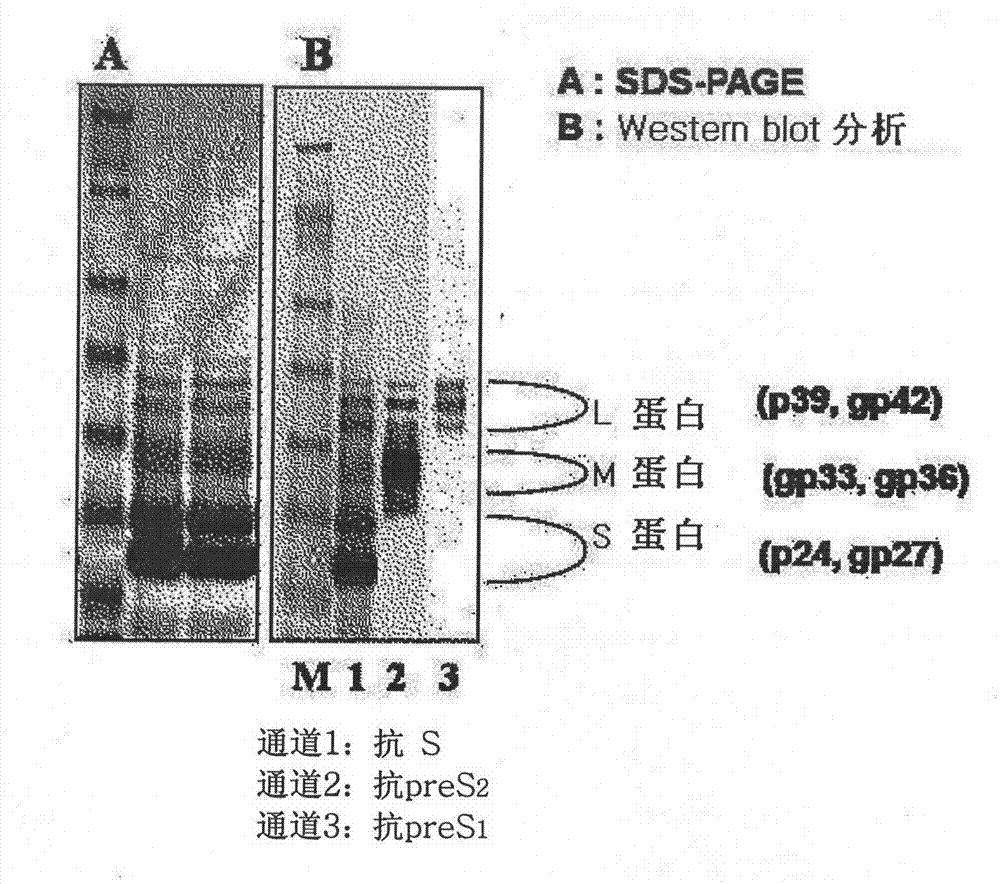
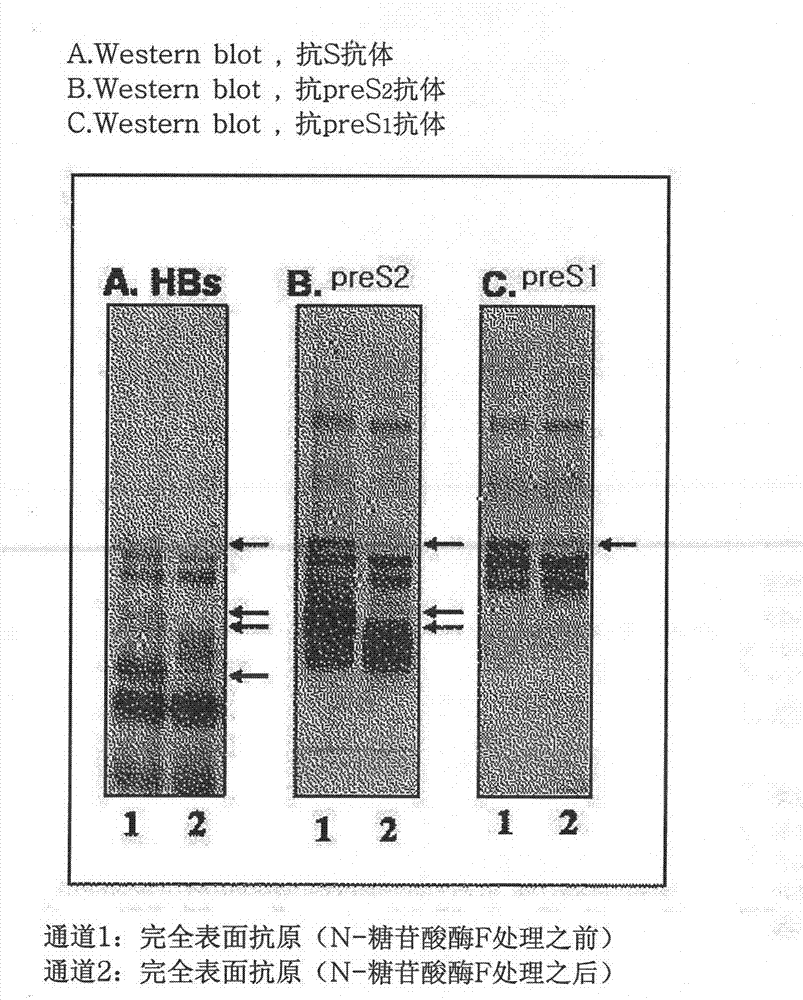
[0087] 1.1 Preparation of recombinant complete surface antigen (preS antigen and S antigen; L-HBsAg)
[0088] (1)-1 clone
[0089] Polymerase chain reaction (PCR) was used as a template to amplify the envelope gene (preS1-preS2-S) and a poly The complete 3'-UTR coding region (SEQ ID NO. 1) of the polyadenylation site was then introduced into an expression vector. At this time, polymerase chain reaction was performed by using a Pfu DNA polymerase, and primers were prepared to amplify the coding region of HBsAg and the complete 3'-UTR [forward primer: 5-GGA AGA TCT CAA TCT CGG GAA-3 (SEQ ID NO.2), reverse primer: 5-GGA AGA TCT CGA ATA GAA GGAAAG-3 (SEQ ID NO.3), the recognition sequence of BglII is underlined]. A polymerase chain reaction product of about 2.75 kbp was obtained, which was coordinated to a pMSG vector linearized by BglII enzyme (see Korean Patent Application No. 10-2000-0043996 and PCT / KR01 / 01285). ...
example 2
[0105] Comparison of Example 2 Recombinant Complete Surface Antigen (L-HBsAg) Immunogenicity
[0106] To determine whether the recombinant whole surface antigen (L-HBsAg) of the present invention is highly immunogenic and induces a strong immune response, animal experiments were conducted to compare with known second-generation hepatitis B vaccines.
[0107] 2.1 Comparison of immunogenicity
[0108] (1) Purpose: To compare the antibody titer and immune response induced by the recombinant L-HBsAg antigen.
[0109] (2) Materials and methods
[0110] (a) Experimental animals: 6-week-old female C57BL / 6 mice.
[0111] (b) Experimental vaccines
[0112] The entire surface antigen (L-HBsAg) prepared and purified according to Example 1-1 was adsorbed on alum to prepare a test vaccine. As a control group, a recombinant S antigen (Hepavax-Gene, Green Cross Co.) produced by Hansenula polymorpha, and a recombinant S antigen without preS antigen (recombinant type B) produced in CHO cel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com