Anti-hepatitis B virus surface antigen completely humanized human antibody and use thereof
A fully human antibody and surface antigen technology, applied in antiviral agents, antiviral immunoglobulins, antibodies, etc., can solve the problems of uncertain risk factors, limited production methods, and complex components of hepatitis B immunoglobulins. The effect of low immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1: Anti-HBV surface antigen fully human antibody A 3 D. 5 preparation of
[0030] 1. Cloning of antibody light chain constant region and heavy chain constant region genes and construction of their vectors
[0031] Isolate healthy human lymphocytes with lymphocyte separation medium, extract total RNA with Trizol reagent, design primers according to the sequences reported in literature [4] and literature [5], and use QIAGEN OneStep RT-PCRKit to amplify the heavy chain and light chain constant regions of human antibodies Gene. The heavy chain and light chain constant region genes of the human antibody were respectively connected to the expression vector AbVec plasmid, and the antibody heavy chain constant region nucleotide vector IgG-AbVec plasmid and the antibody light chain constant region nucleotide vector Igκ-AbVec were respectively constructed Plasmid, after sequencing verification, it was confirmed that the correct clone was obtained. Wherein, the nucleotid...
Embodiment 2
[0040] Example 2: Anti-HBV surface antigen fully human antibody A 3 D. 5 specificity
[0041] Detection of fully human monoclonal antibody A by Elisa method 3 D. 5 Specifically binds to HBsAg protein.
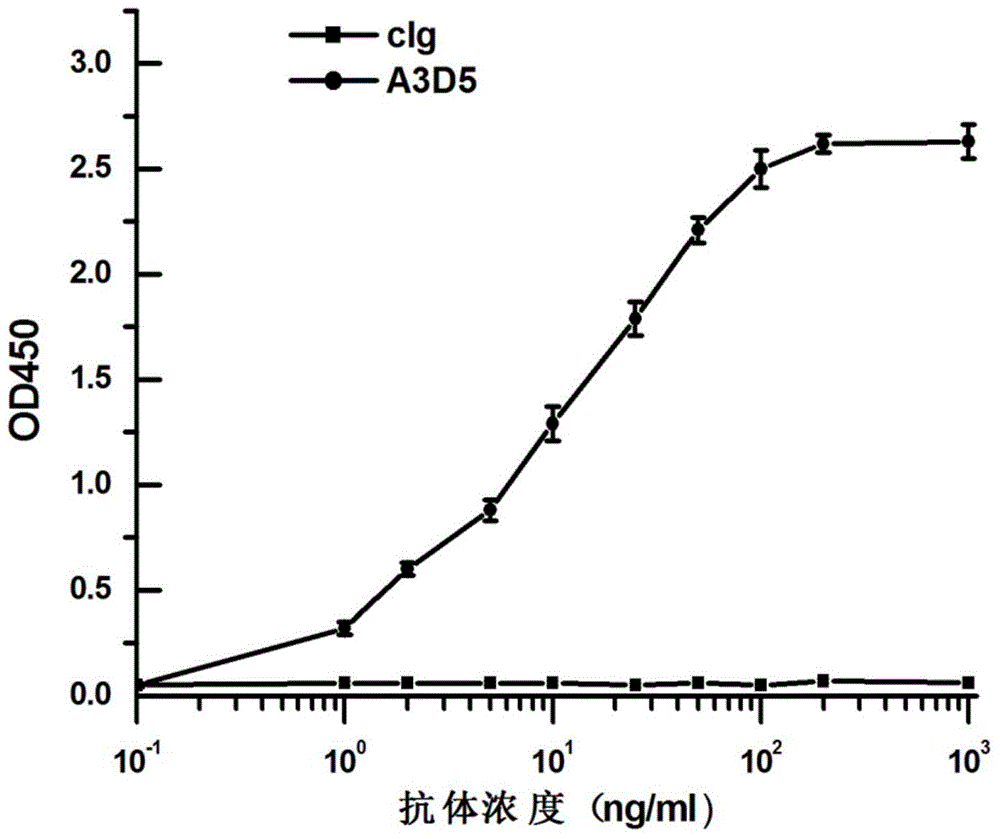
[0042] The recombinant HBsAg protein and the control protein cIg were coated on the ELISA plate, and after being blocked by the blocking solution, the blank group, different concentrations of the control protein cIg, and different concentrations of expressed and purified A 3 D. 5 Antibody incubation, adding Goatanti-humankappa-HRP after washing the plate, TMB chromogenic solution for color development, microplate reader reads at 450nm wavelength.
[0043] Draw according to antibody concentration and corresponding OD value figure 1 ,Depend on figure 1 The analysis shows that with the increase of the antibody concentration, the corresponding reading value of the HBsAg protein group is also correspondingly increased. However, the reading value of the coated control protein...
Embodiment 3
[0044] Example 3: Anti-HBV surface antigen fully human antibody A 3 D. 5 Binding epitope identification
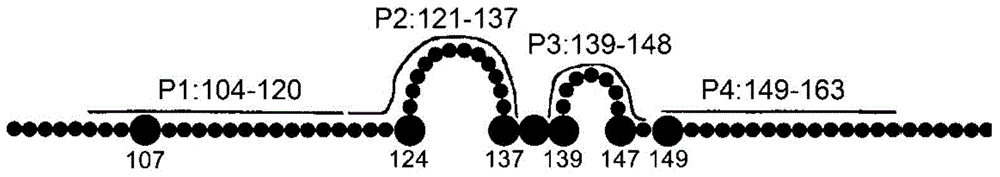
[0045] Combining the antigenic epitope region of hepatitis B surface antigen (HBsAg) with neutralizing activity reported in previous literature, a biotin-labeled short peptide of hepatitis B surface antigen was synthesized to measure the binding of fully human hepatitis B surface protein monoclonal antibody on HBsAg area. Such as figure 2 As shown in the HBsAg pattern diagram, the synthetic four biotin-labeled HBsAg short peptide P 1 (aa:104-120), P 2 (aa:121-137), P 3 (aa:139-148), P 4 (aa:149-163), the amino acid sequences of P1-P4 are shown in SEQ ID NO.9-SEQ ID NO.12. Among them, the antigenic short peptide P 1 and P 4 is a linear structure, P 2 and P 3 ring structure [6] .
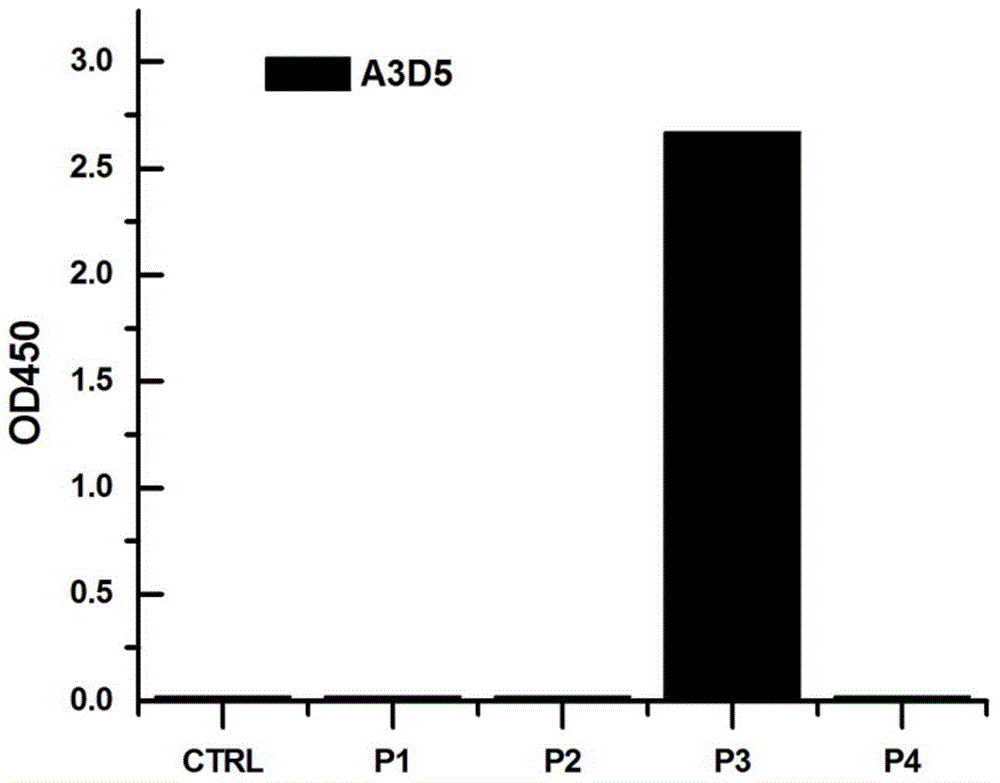
[0046] A was analyzed by ELISA 3 D. 5 The binding epitope of the monoclonal antibody, composed of image 3 It can be seen that A 3 D. 5 Antibody binds to P 3 Location.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com