Human anti-HBV surface antigen genetic engineering antibody, and preparation method and application thereof
A technology of genetically engineered antibodies and surface antigens, applied in genetic engineering, antiviral agents, antiviral immunoglobulins, etc., can solve problems such as mass production limitations, limited applications, and infections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1 Materials and methods
[0033] 1. Viruses, cells, and carriers: Hepatitis B virus HBV was provided by the Hepatitis Department of the Institute of Viral Disease Prevention and Control, Chinese Center for Disease Control and Prevention. Human liver cancer cell line HepG2 (ATCC); insect cells Sf9 were from American Cell Culture Center (ATCC). The strain is XLI-Blue (Stratagene, USA), and the vector is pComb3H (40kb), donated by Scripps Research Institute, USA. Baculovirus expression vector is pAC-K-Fc (German PROGEN PR3003) (Liang, M.F., Stefan, D., Li, D.X., Queitsch, I., Li, W., and Bautz, E.F. Baculovirus expression cassette vectors for rapid production of complete human IgG from phage displayselected antibody fragments. Journal of Immunological Methods. 247:119-130). Hepatitis B surface antigen (CHO-HBsAg) was purchased from Beijing Wantai Biological Pharmaceutical Company; mouse anti-hepatitis B surface antigen monoclonal antibody 3E7 (Mouse Anti-Hepat...
Embodiment 2
[0060] Embodiment 2 result and analysis
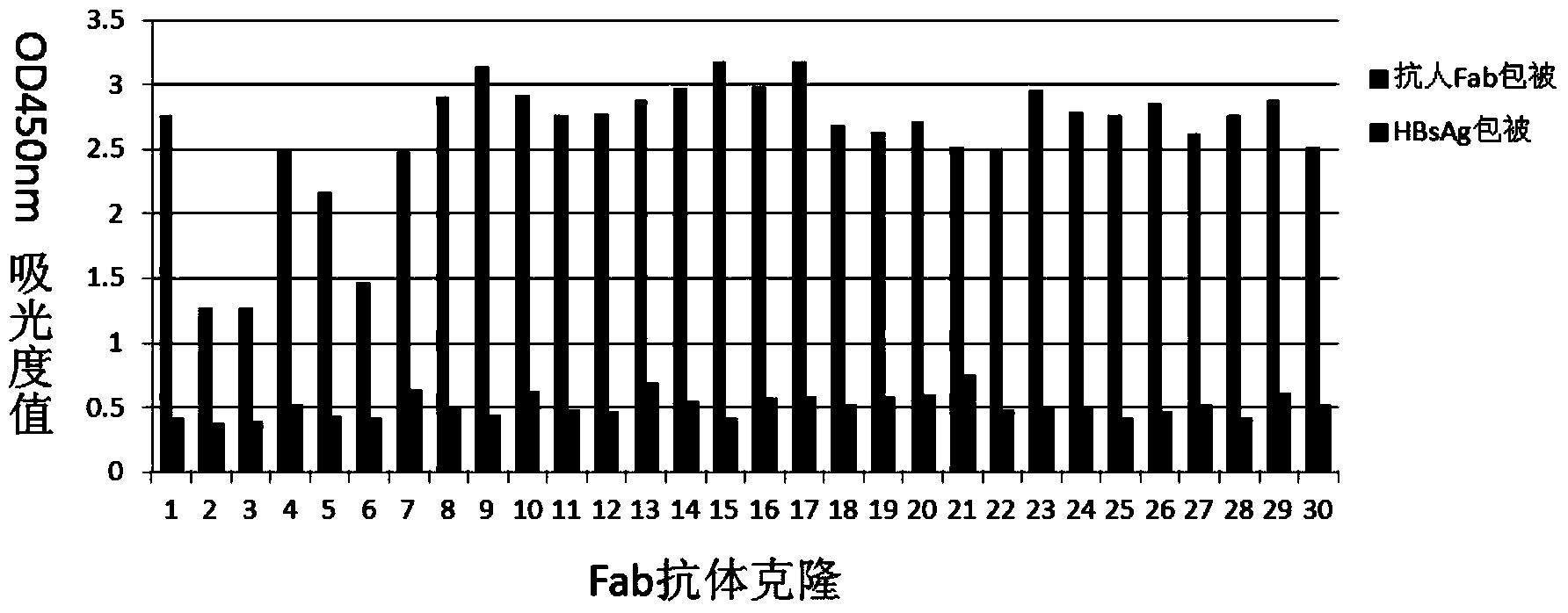
[0061] 1. Screening of human anti-HBsAg antibody library
[0062] Successfully built 4 Kappa libraries and 4 Lambda libraries, the library capacity is 1×10 7 Above, the light and heavy chain insertion rate is above 95%. The Kappa sub-library and the Lambda sub-library were packaged separately. After three rounds of screening with purified hepatitis B surface antigen, it was found that the Kappa sub-library was enriched in three rounds of screening, and the capacity of the eluted library gradually increased, while the Lambda sub-library did not appear. Pick 100 Kappa sub-bank clones and 100 lambda sub-bank clones, ELISA results showed that there were 30 HBsAg-positive clones, all from the Kappa sub-bank, and 10 antibody clones with different sequences were found by sequencing, as shown in Table 2:
[0063] Table 2 Output of antibody library packaging and enrichment screening
[0064]
[0065] 2. Sequence analysis of human anti-HBs...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com