Hepatitis B nucleic acid vaccine and construction method thereof
A nucleic acid vaccine, hepatitis B technology, applied in the field of biomedicine, can solve the problems of unsatisfactory immune prevention effect and treatment effect, unsatisfactory effect, low immune reactivity, etc., and achieves good immune effect, long-lasting immune effect, The effect of simplifying the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
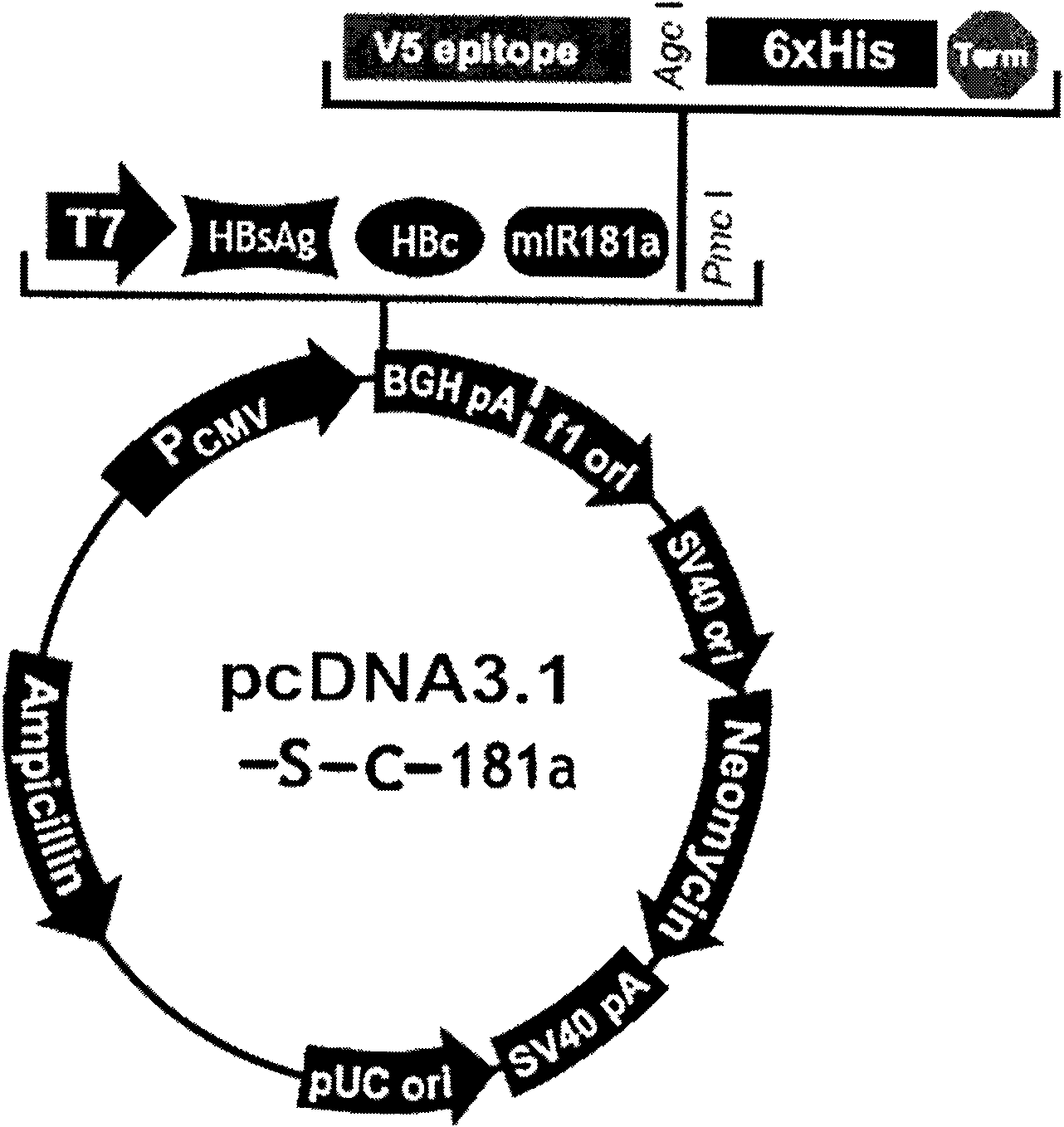
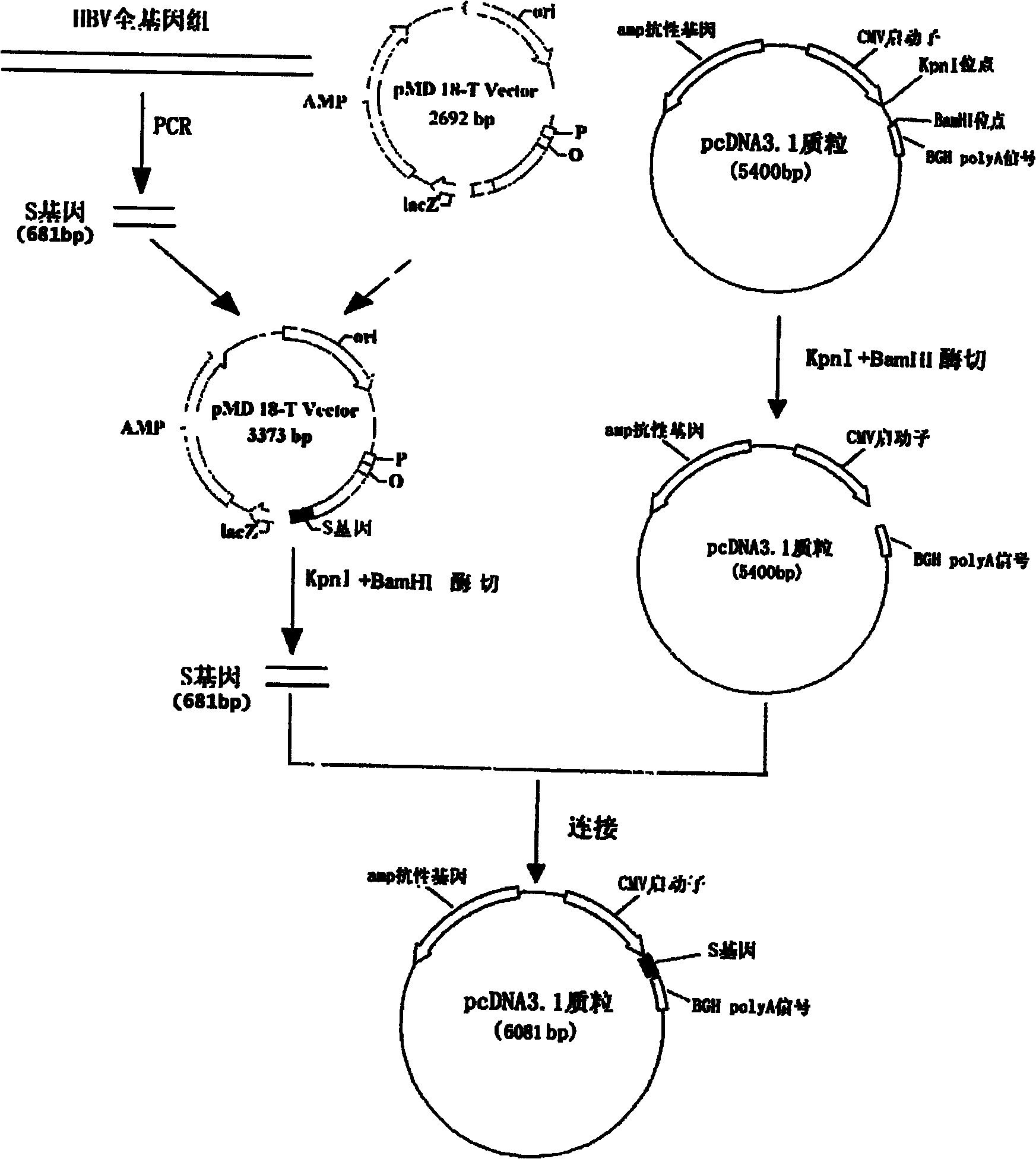
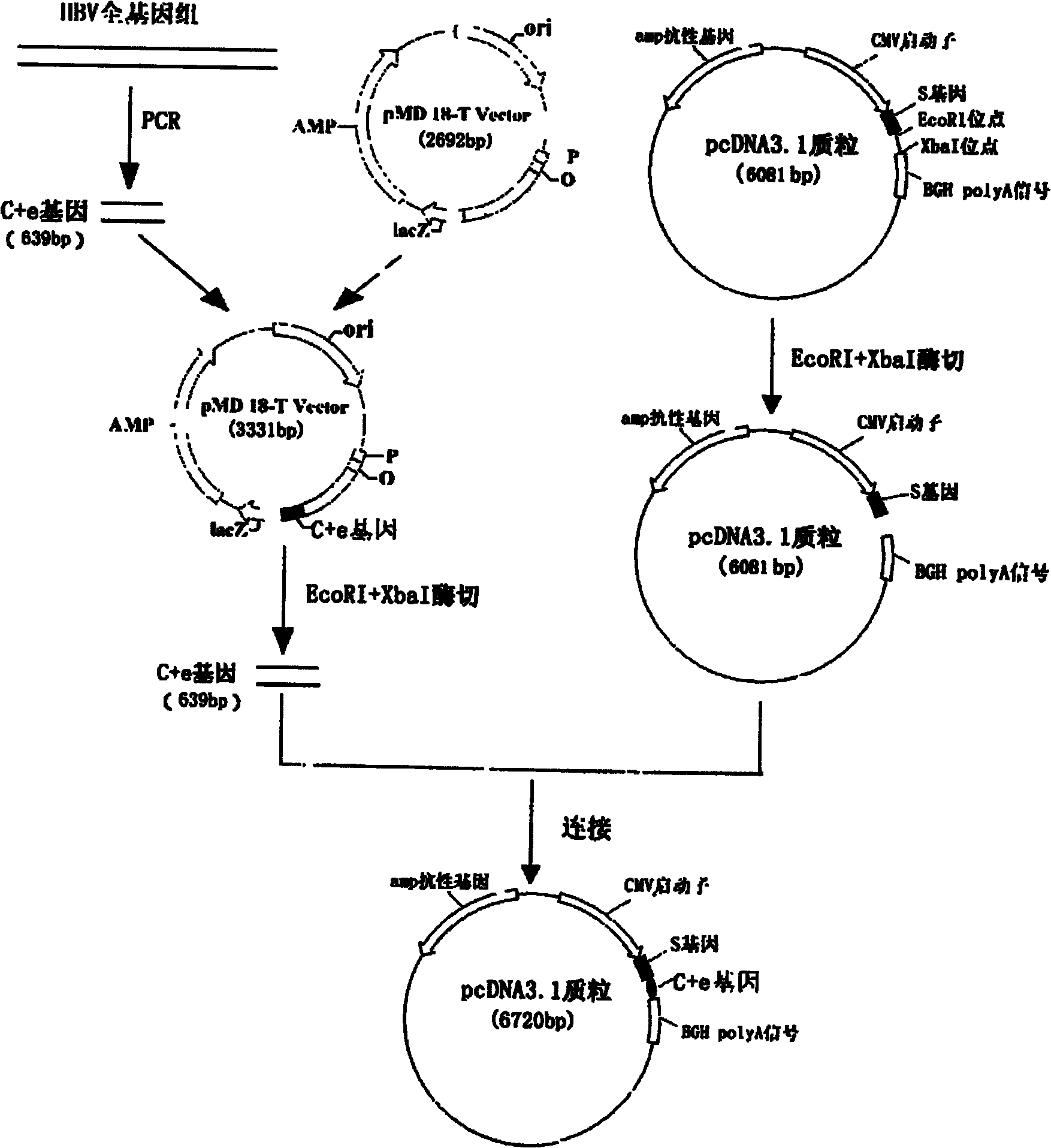
[0030] Example 1: Construction of the nucleic acid vaccine of the present invention
[0031] The DNA vaccine is constructed by molecular biology experimental methods such as PCR, restriction digestion, ligation, transformation, etc., which can be referred to the second edition of "Molecular Cloning" written by Sambrook, USA. The PCR primer design software is Primer PREMER V from Premier Biosoft International.
[0032] The basic materials for constructing this vaccine include: human monocyte THP-1 (purchased from the Cell Bank of the Chinese Academy of Sciences), Hep G 2.215 liver cancer cells stably transfected with HBV whole virus (purchased from the Wuhan Institute of Virology, Chinese Academy of Sciences); eukaryotic expression vector pcDNA TM 3.1 / V5-His B plasmid (purchased from Invitrogen).
[0033] The reagents required for constructing this accounting virus include: Prime Star high-fidelity DNA polymerase (purchased from TaKaRa), pMD18T cloning vector (purchased from TaKaRa),...
Embodiment 2
[0108] Example 2: Animal experiment of the nucleic acid vaccine of the present invention
[0109] In order to verify the protective efficacy of the present invention against HBV infection, the present invention compared pcDNA3.1-SC-181a, pcDNA3.1-S expressing HBV S antigen, and pcDNA3.1-C expressing HBV C antigen and e antigen against mice. The difference in immune effect.
[0110] The experimental mice were female 8-week-old Balb / C mice, 30 of which were divided into 3 groups, 10 in each group. One group was injected with pcDNA3.1-S-C-181a, the second group was injected with pcDNA3.1-S, and the third group was injected with pcDNA3.1-C. The injection method was intramuscular injection, and the injection amount was 0.1 mg / mouse. In order to enhance the immune effect, insert the electric pulse shock vertically after the injection with the injection hole as the center. The electrode needle is inserted slightly deeper than the injection depth, the electric field strength is 200v / cm, t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com