Hepatitis B virus multi-epitope fusion protein and preparation method and application thereof
A technology of hepatitis B virus and fusion protein, which is applied in the field of fusion protein, can solve the problems of poor immunogenicity, difficulty in adapting to long-term treatment of patients with chronic HBV infection, multiple immunizations, and single epitope, so as to enhance the immune effect and solve multiple problems. Second immune tolerance, strong immunogenic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
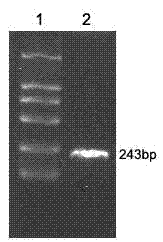
[0028] Embodiment 1, the design and preparation of HBV multi-epitope fusion protein
[0029] 1. Design of HBV multi-epitope fusion protein
[0030] First, use the HBV antigen supertype epitope database established by the Institute of Immunology of the Chinese People’s Liberation Army to screen the supertype epitopes that meet the following criteria: ①The antigen is HBsAg, HBcAg or pol; ②MHC is restricted to A2, A3 supertype; ③ The length of the epitope was 9 peptides or 10 peptides; ④ The ability of inducing specific CTL to secrete cytokine IFN-γ in vitro was higher than that of the control epitope HBcAg18-27. As a result, the following four supertype epitopes were screened out, namely:
[0031] HBsAg313-321 (amino acid sequence is IPIPSSWAF), MHC restricted to A3 supertype (including A0301, A1101, A3301);
[0032] HBsAg335-343 (amino acid sequence is WLSLLVPFV), MHC restricted to A2 supertype (including A0201, A0202, A0203, A0206 and A6802);
[0033] Pol150-159 (amino ...
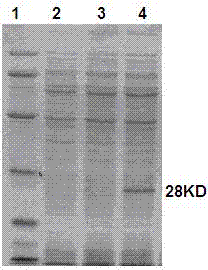
Embodiment 2
[0044] Example 2, the immune protection of HBV multi-epitope fusion protein
[0045] F1 HBV generation after hybridization of immunized A2, A3 transgenic mice (purchased from Jackson Laboratory) and HBV transgenic mice (purchased from Guangzhou Air Force Hospital) + A2 + , HBV + A3 + 60 transgenic C57 mice were divided into 6 groups on average: HBV multi-epitope fusion protein immunization group, HBc particle-HBV multi-epitope fusion peptide mixed immunization control group, HBc particle immunization control group, HBV multi-epitope fusion peptide immunization control group group, non-treatment control group, and placebo control group. The immunization method is as follows: HBV multi-epitope fusion protein immunization group: HBV multi-epitope fusion protein 100 μg / time / animal, 1 time / week; HBc particles-HBV multi-epitope fusion peptide mixed immunization control group: HBc particles and HBV multi-epitope fusion peptide Mixture of fusion peptides 100 μg / time / mouse, 1 tim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com