Recombinant vaccinia virus of Tiantan strain with IFN-gamma receptor gene (B8R) deletion and applications thereof
A vaccinia virus and gene deletion technology, which is applied in antiviral agents, genetic engineering, plant gene improvement, etc., can solve the problems of inability to induce CTL response, inability to protect HIV-1 attack, etc., and achieve the goal of improving safety and immunogenicity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
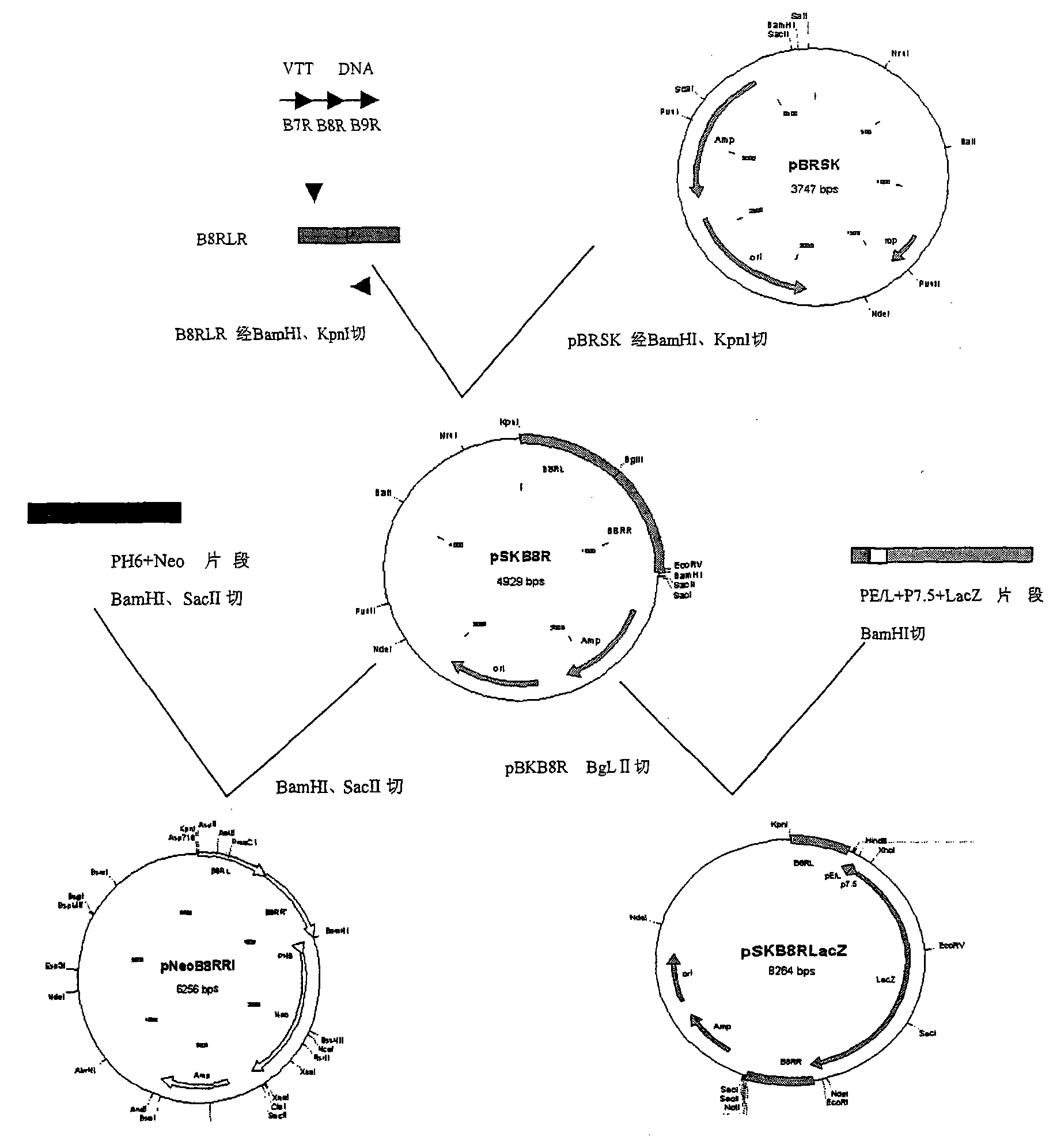
[0038] Embodiment one, the construction of transfer plasmid
[0039] 1. Construction of plasmid pBR-SK
[0040] 5'PBR322-SACI-FOR
[0041] G GAGCTC CGACCGATGCCCTTGAGAGCC
[0042] 3'PBR322-KPNI-RE
[0043] GG GGTACC AGGTGGCACTTTTCGGGGAAATG
[0044] PCR amplified commercial plasmid pBR322 including the sequence of all functional elements, denoted as pBR.
[0045] reaction system
[0046] 10×Pyrobest Buffer 5μl
[0047] dNTPMisture (2.5mM each) 5μl
[0048] 3'PBR322-KPNI-RE (50μM) 0.5μl
[0049] 5'PBR322-SACI-FOR (50μM) 0.5μl
[0050] pBR322 0.5 μl
[0051] Pyrobest DNA Polymerase (5U / ml) 0.5μl
[0052] ddH2O 38μl
[0053] Reaction conditions Pre-denaturation at 94°C for 2 minutes; 94°C for 30 s, 68°C for 5 min, a total of 35 cycles; 72°C for 7 min; 4°C.
[0054] The PCR product pBR was purified and co-digested with KpnI and SacI of Dalian Biotech, and recovered by running gel.
[0055] The plasmid pBS-SK was co-digested with KpnI and SacI of Dalian Baobiology to...
Embodiment 3 8
[0108] Example 3 B8R gene deletion expresses HIV-1 antigen VTKgpeΔB8RlacZ (B8R gene deletion is replaced by lacZ, TK region is inserted into B' / C type CN54 strain HIV-1 gagpol env gene) AIDS vaccine of Tiantan strain recombinant vaccinia virus.
[0109] VTKgpe (Tiantan strain recombinant vaccinia virus whose TK region is inserted into the B' / C type CN54 strain HIV-1 gagpol env gene) and the transfer plasmid pSKB8RLacZ were co-transfected into CEF cells for homologous recombination. After blue-white screening, continuous single-spot purification, The recombinant virus VTKgpeΔB8RlacZ with the deletion of B8R gene replaced by lacZ was identified. After infecting 80% of CEF cells in a sheet with 0.1-0.01 PFU / cell virus amount VTKgpe and adsorbing for 1-1.5 hours, the recombinant plasmid pSKB8RLacZ was transfected into CEF cells using liposome transfection technology (refer to the Lipofectin kit from INVITROGEN, USA). After 48 hours, the recombinant virus liquid was harvested, and ...
Embodiment 4
[0110] Example 4 AIDS vaccine of Tiantan strain recombinant vaccinia virus with B8R gene deletion and HIV-1 antigen-expressing VTKgpeΔB8R (B8R gene deletion without lacZ selection marker, TK region inserted into B' / C type CN54 strain HIV-1 gagpol env gene) .
[0111] Then, VTKgpeΔB8RlacZ was used as the parent strain to co-transfect CEF cells with pSKB8RNeo for homologous recombination. After G418 pressure screening, blue-white screening, continuous single-spot purification, and identification of the recombinant virus VTKgpeΔB8R with B8R gene deletion and no lacZ selection marker. After infecting 80% of CEF cells in a sheet with 0.1-0.01 PFU / cell virus amount VTKgpeΔB8RlacZ for 1-1.5 hours, the recombinant plasmid pSKB8RNeo was transfected into CEF cells using liposome transfection technology (refer to the Lipofectin kit from INVITROGEN, USA). After 48 hours, the recombinant virus fluid was harvested, and pressure-transferred with 400ug / ml G418 for three generations. Use nutr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com