Therapeutic hepatitis B vaccine based on HBV PreS-S, C antigen and novel adjuvant CpG
A technology of hepatitis B surface antigen and hepatitis B core antigen, which is applied in the field of hepatitis B vaccine, can solve the problems of no treatment disease, no clear proposal for the transformation of anti-HBc antibody subtypes, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
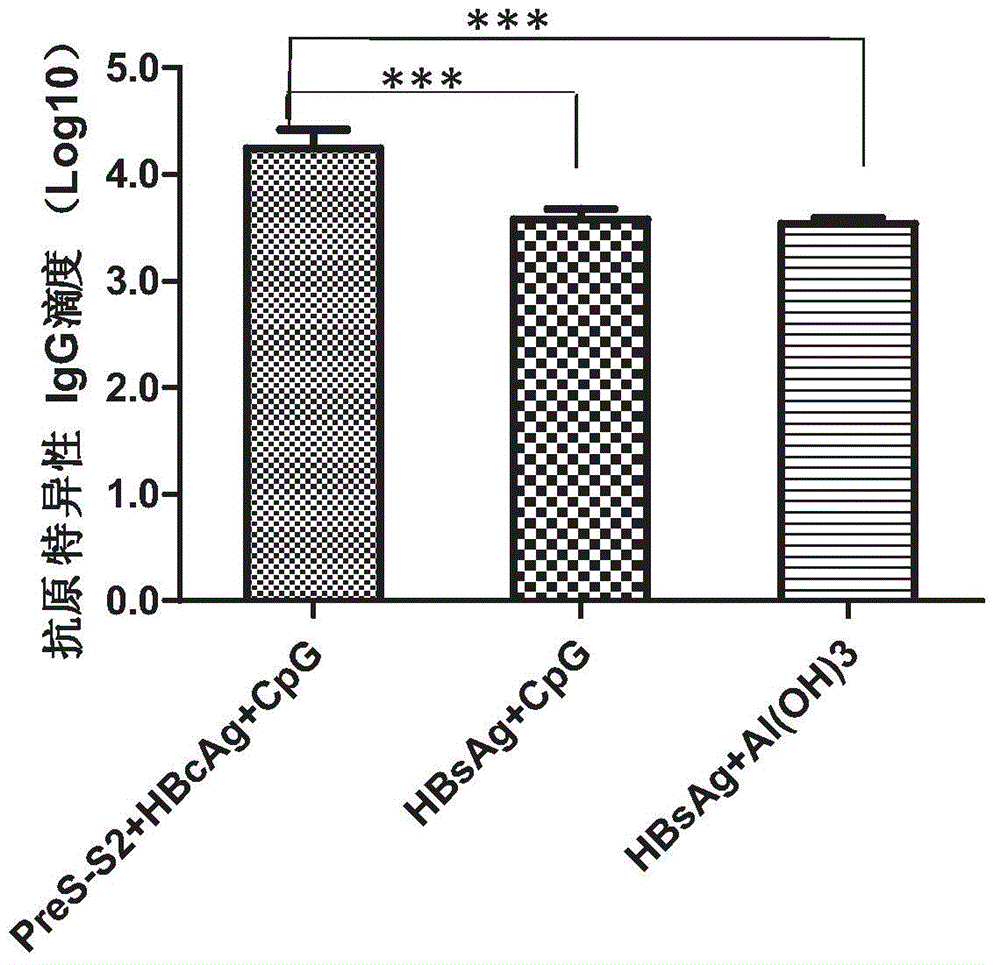
[0079] Example 1. Hepatitis B surface antigen (HBsAg, S) and its precursor PreS2 are combined with hepatitis B core antigen (HBcAg) and CpG-ODN to enhance the immune response of hepatitis B surface antigen total IgG.
[0080] In order to detect the total IgG immune response of Hepatitis B surface antigen of the PreS2-S+HBcAg+CpG-ODN composition, the present inventors respectively used PreS2-S, HBcAg and CpG-ODN, HBsAg and CpG-ODG, HBsAg and Al(OH) 3 The adjuvant was mixed, and mice were immunized with the adjuvant. The HBsAg specific IgG level in the serum was measured and statistical analysis was performed to evaluate the relative HBsAg and CpG-ODG, HBsAg and Al(OH) 3 Combination, PreS2-S, HBcAg and CpG-ODN combined use on HBsAg total IgG immune response.
[0081] In this example, C57BL / 6 mice, female, 6-8 weeks old, were purchased from Shanghai Slack Company. The PreS2-S antigen used in this example was prepared by the inventors and is a natural PreS2-S expressed by Hansenula yeas...
Embodiment 2
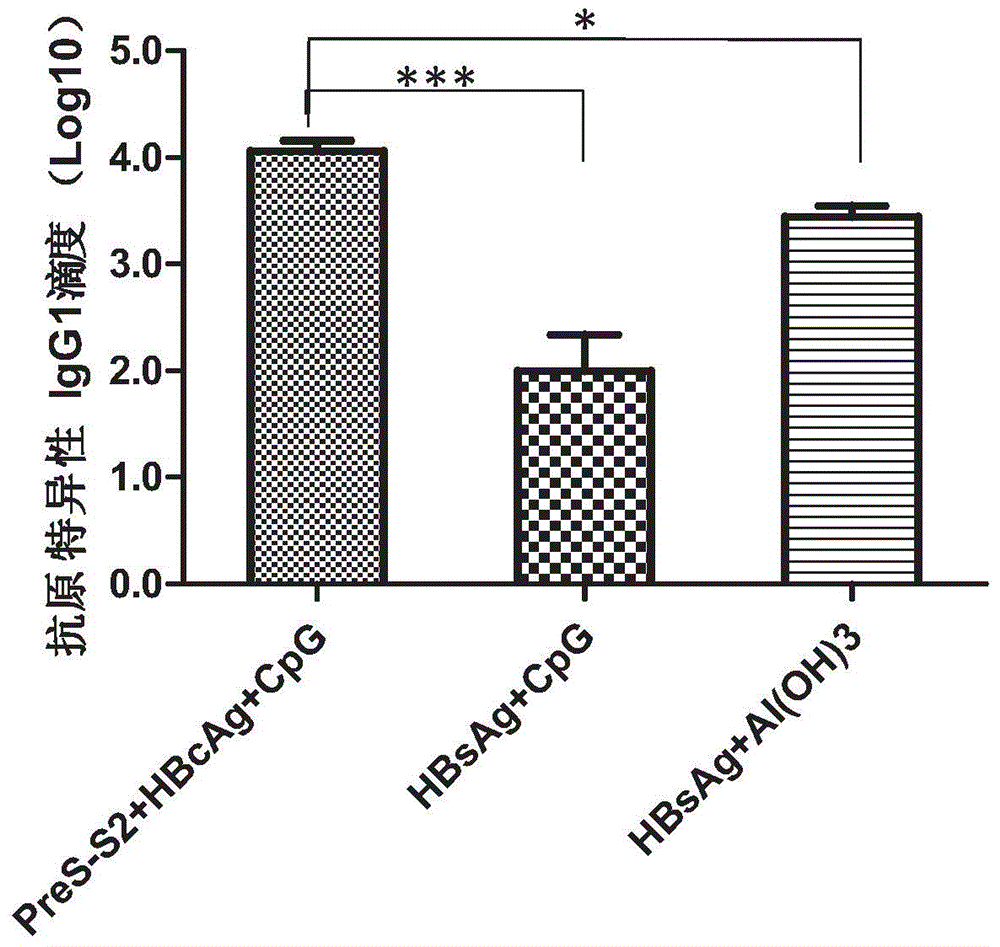
[0085] Example 2. The combined use of hepatitis B surface antigen and its precursors PreS (PreS2-S), hepatitis B core antigen (HBcAg) and CpG-ODN at the level of humoral immunity enhances the hepatitis B surface antigen Th2 immune response.
[0086] According to the method described in Example 1, the effect of the PreS2-S+HBcAg+CpG-ODN composition of the present invention on the Th2 immune response of mice was determined. The difference is that the enzyme-labeled antibody used in the detection is horseradish peroxidation Enzyme-labeled goat anti-mouse IgG1 (purchased from SouthernBiotech, USA), the dilution factor is 1:20000. The results are shown in figure 2 in.
[0087] Antigen-specific immune responses are divided into two types, Th1 and Th2, and Th2 type responses correspond to high levels of antigen-specific IgG1 antibody titer. Al(OH) 3 It is a very strong Th2 vaccine adjuvant that can inhibit Th1 immune response, which is manifested by the induction of high levels of speci...
Embodiment 3
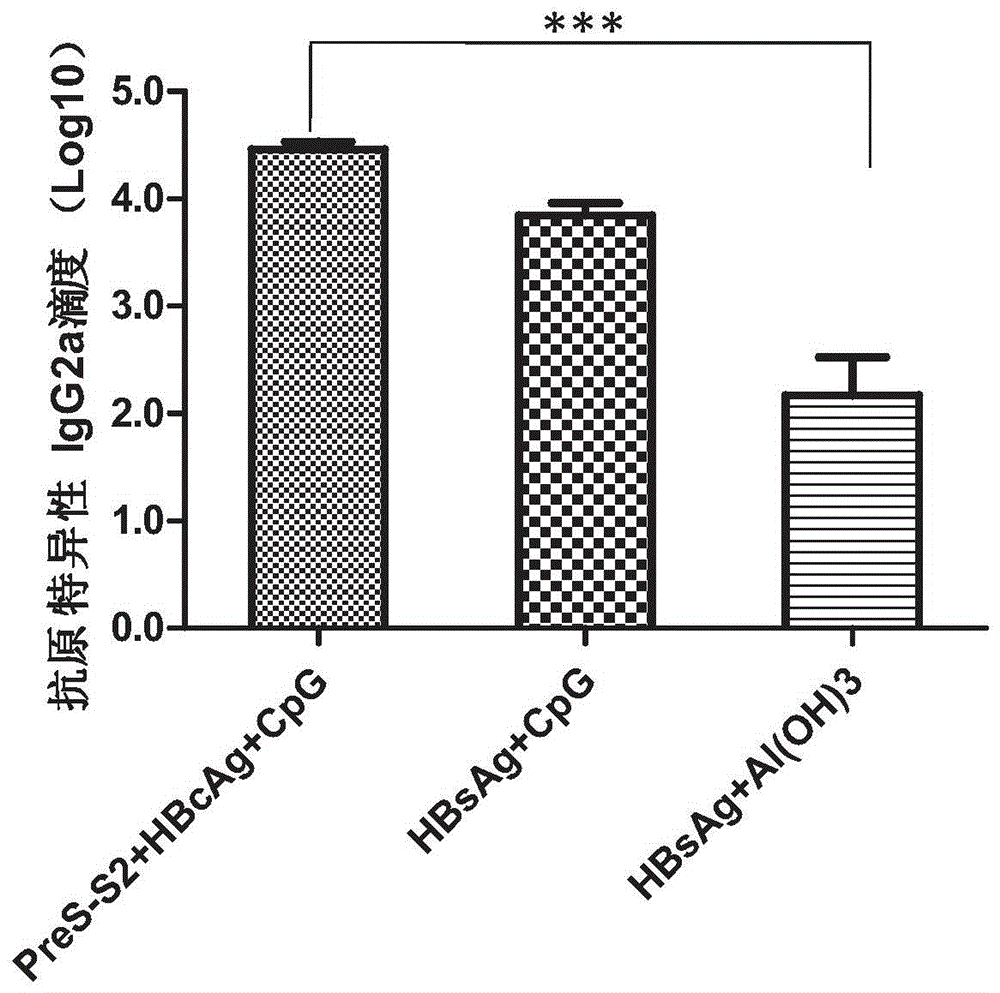
[0088] Example 3. The combined use of hepatitis B surface antigen and its precursor PreS (PreS2-S), hepatitis B core antigen (HBcAg) and CpG-ODN at the level of humoral immunity enhances the immune response of hepatitis B surface antigen Th1.
[0089] According to the method described in Example 1, the effect of the PreS2-S+HBcAg+CpG-ODN composition of the present invention on the Th1 immune response of mice was determined. The difference is that the enzyme-labeled antibody used in the detection is horseradish peroxidation Enzyme-labeled goat anti-mouse IgG2a (purchased from SouthernBiotech, USA), the dilution ratio is 1:6000. The results are shown in image 3 in.
[0090] As mentioned in Example 2, Al(OH) 3 It is a very strong Th2 vaccine adjuvant that can inhibit Th1 immune response, which is manifested by the induction of very low levels of specific IgG2a antibodies after immunization. CpG-ODG is a strong Th1 vaccine adjuvant, which can enhance Th1 immune response, which is exp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com